Molecular characterization of a heteromeric ATP-citrate lyase that generates cytosolic acetyl-coenzyme A in Arabidopsis
- PMID: 12376641
- PMCID: PMC166603
- DOI: 10.1104/pp.008110
Molecular characterization of a heteromeric ATP-citrate lyase that generates cytosolic acetyl-coenzyme A in Arabidopsis
Abstract
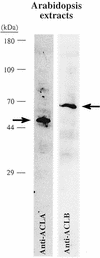
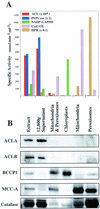
Acetyl-coenzyme A (CoA) is used in the cytosol of plant cells for the synthesis of a diverse set of phytochemicals including waxes, isoprenoids, stilbenes, and flavonoids. The source of cytosolic acetyl-CoA is unclear. We identified two Arabidopsis cDNAs that encode proteins similar to the amino and carboxy portions of human ATP-citrate lyase (ACL). Coexpression of these cDNAs in yeast (Saccharomyces cerevisiae) confers ACL activity, indicating that both the Arabidopsis genes are required for ACL activity. Arabidopsis ACL is a heteromeric enzyme composed of two distinct subunits, ACLA (45 kD) and ACLB (65 kD). The holoprotein has a molecular mass of 500 kD, which corresponds to a heterooctomer with an A(4)B(4) configuration. ACL activity and the ACLA and ACLB polypeptides are located in the cytosol, consistent with the lack of targeting peptides in the ACLA and ACLB sequences. In the Arabidopsis genome, three genes encode for the ACLA subunit (ACLA-1, At1g10670; ACLA-2, At1g60810; and ACLA-3, At1g09430), and two genes encode the ACLB subunit (ACLB-1, At3g06650 and ACLB-2, At5g49460). The ACLA and ACLB mRNAs accumulate in coordinated spatial and temporal patterns during plant development. This complex accumulation pattern is consistent with the predicted physiological needs for cytosolic acetyl-CoA, and is closely coordinated with the accumulation pattern of cytosolic acetyl-CoA carboxylase, an enzyme using cytosolic acetyl-CoA as a substrate. Taken together, these results indicate that ACL, encoded by the ACLA and ACLB genes of Arabidopsis, generates cytosolic acetyl-CoA. The heteromeric organization of this enzyme is common to green plants (including Chlorophyceae, Marchantimorpha, Bryopsida, Pinaceae, monocotyledons, and eudicots), species of fungi, Glaucophytes, Chlamydomonas, and prokaryotes. In contrast, all known animal ACL enzymes have a homomeric structure, indicating that a evolutionary fusion of the ACLA and ACLB genes probably occurred early in the evolutionary history of this kingdom.
Figures







References
-
- Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. - PubMed
-
- Anderson M, Roberts JA. Arabidopsis: Annual Plant Reviews. Vol. 1. Boca Raton, FL: CRC Press; 1998.
-
- Arnold GW, Hill JJ. Chemical factors affecting the selection of food plants by ruminants. In: Harborne J, editor. Phytochemical Ecology. New York: Academic Press; 1972. pp. 71–101.
-
- Back K, He S, Kim KU, Shin DH. Cloning and bacterial expression of sesquiterpene cyclase, a key branch point enzyme for the synthesis of sesquiterpenoid phytoalexin capsidiol in UV-challenged leaves of Capsicum annuum. Plant Cell Physiol. 1998;39:899–904. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

