Cajal body-specific small nuclear RNAs: a novel class of 2'-O-methylation and pseudouridylation guide RNAs
- PMID: 12032087
- PMCID: PMC126017
- DOI: 10.1093/emboj/21.11.2746
Cajal body-specific small nuclear RNAs: a novel class of 2'-O-methylation and pseudouridylation guide RNAs
Abstract
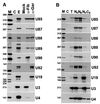
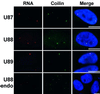
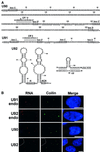
Cajal (coiled) bodies are conserved subnuclear organelles that are present in the nucleoplasm of both animal and plant cells. Although Cajal bodies were first described nearly 100 years ago, their function has remained largely speculative. Here, we describe a novel class of human small nuclear RNAs that localize specifically to Cajal bodies. The small Cajal body-specific RNAs (scaRNAs) are predicted or have already been demonstrated to function as guide RNAs in site-specific synthesis of 2'-O-ribose-methylated nucleotides and pseudouridines in the RNA polymerase II-transcribed U1, U2, U4 and U5 spliceosomal small nuclear RNAs (snRNAs). Our results provide strong support for the idea that the Cajal body, this mysterious nuclear organelle, provides the cellular locale for post-transcriptional modification of spliceosomal snRNAs.
Figures

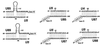

References
-
- Balakin A.G., Smith,L. and Fournier,M.J. (1996) The RNA world of the nucleolus: two major families of small RNAs defined by different box elements with related functions. Cell, 86, 823–834. - PubMed
-
- Bohmann K., Ferreira,J., Santama,N., Weis,K. and Lamond,A.I. (1995) Molecular analysis of the coiled body. J. Cell Sci., 19, Suppl., 107–113. - PubMed
-
- Burge C.B., Tuschl,T. and Sharp,P.A. (1999) Splicing of precursors to mRNAs by the spliceosome. In Gesteland,R.F., Cech,T.R. and Atkins,J.F. (eds), The RNA World. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY, pp. 525–560.
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources

