The regulation of actin organization by actin-depolymerizing factor in elongating pollen tubes
- PMID: 12215514
- PMCID: PMC150764
- DOI: 10.1105/tpc.003038
The regulation of actin organization by actin-depolymerizing factor in elongating pollen tubes
Abstract
Pollen tube elongation is a polarized cell growth process that transports the male gametes from the stigma to the ovary for fertilization inside the ovules. Actomyosin-driven intracellular trafficking and active actin remodeling in the apical and subapical regions of pollen tubes are both important aspects of this rapid tip growth process. Actin-depolymerizing factor (ADF) and cofilin are actin binding proteins that enhance the depolymerization of microfilaments at their minus, or slow-growing, ends. A pollen-specific ADF from tobacco, NtADF1, was used to dissect the role of ADF in pollen tube growth. Overexpression of NtADF1 resulted in the reduction of fine, axially oriented actin cables in transformed pollen tubes and in the inhibition of pollen tube growth in a dose-dependent manner. Thus, the proper regulation of actin turnover by NtADF1 is critical for pollen tube growth. When expressed at a moderate level in pollen tubes elongating in in vitro cultures, green fluorescent protein (GFP)-tagged NtADF1 (GFP-NtADF1) associated predominantly with a subapical actin mesh composed of short actin filaments and with long actin cables in the shank. Similar labeling patterns were observed for GFP-NtADF1-expressing pollen tubes elongating within the pistil. A Ser-6-to-Asp conversion abolished the interaction between NtADF1 and F-actin in elongating pollen tubes and reduced its inhibitory effect on pollen tube growth significantly, suggesting that phosphorylation at Ser-6 may be a prominent regulatory mechanism for this pollen ADF. As with some ADF/cofilin, the in vitro actin-depolymerizing activity of recombinant NtADF1 was enhanced by slightly alkaline conditions. Because a pH gradient is known to exist in the apical region of elongating pollen tubes, it seems plausible that the in vivo actin-depolymerizing activity of NtADF1, and thus its contribution to actin dynamics, may be regulated spatially by differential H(+) concentrations in the apical region of elongating pollen tubes.
Figures



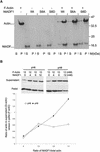
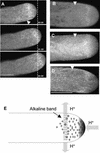
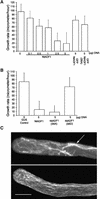

References
-
- Agnew, B.J., Minamide, L.S., and Bamburg, J.R. (1995). Reactivation of phosphorylated actin depolymerizing factor and identification of the regulatory site. J. Biol. Chem. 270, 17582–17587. - PubMed
-
- Aizawa, H., Fukui, Y., and Yahara, I. (1997). Live dynamics of Dictyostelium cofilin suggests a role in remodeling actin latticework into bundles. J. Cell Sci. 110, 2333–2344. - PubMed
-
- Ausubel, F.M., Brent, R., Kingston, R.E., Moore, D.D., Seidman, J.G., Smith, J.A., and Struhl, K. (2000). Current Protocols in Molecular Biology. (New York: John Wiley & Sons).
-
- Bamburg, J.R. (1999). Proteins of the ADF/cofilin family: Essential regulators of actin dynamics. Annu. Rev. Cell Biol. 15, 185–230. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

