Sequence analysis of a functional Drosophila centromere
- PMID: 12566396
- PMCID: PMC420369
- DOI: 10.1101/gr.681703
Sequence analysis of a functional Drosophila centromere
Abstract
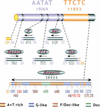
Centromeres are the site for kinetochore formation and spindle attachment and are embedded in heterochromatin in most eukaryotes. The repeat-rich nature of heterochromatin has hindered obtaining a detailed understanding of the composition and organization of heterochromatic and centromeric DNA sequences. Here, we report the results of extensive sequence analysis of a fully functional centromere present in the Drosophila Dp1187 minichromosome. Approximately 8.4% (31 kb) of the highly repeated satellite DNA (AATAT and TTCTC) was sequenced, representing the largest data set of Drosophila satellite DNA sequence to date. Sequence analysis revealed that the orientation of the arrays is uniform and that individual repeats within the arrays mostly differ by rare, single-base polymorphisms. The entire complex DNA component of this centromere (69.7 kb) was sequenced and assembled. The 39-kb "complex island" Maupiti contains long stretches of a complex A+T rich repeat interspersed with transposon fragments, and most of these elements are organized as direct repeats. Surprisingly, five single, intact transposons are directly inserted at different locations in the AATAT satellite arrays. We find no evidence for centromere-specific sequences within this centromere, providing further evidence for sequence-independent, epigenetic determination of centromere identity and function in higher eukaryotes. Our results also demonstrate that the sequence composition and organization of large regions of centric heterochromatin can be determined, despite the presence of repeated DNA.
Figures





References
-
- The Arabidopsis Genome Initiative, 2000. Analysis of the genome sequence of the flowering plant Arabidopsis thaliana. In Nature, pp. 796–815. - PubMed
-
- Adams M.D., Celniker, S.E., Holt, R.A., Evans, C.A., Gocayne, J.D., Amanatides, P.G., Scherer, S.E., Li, P.W., Hoskins, R.A., Galle, R.F., et al. 2000. The genome sequence of Drosophila melanogaster. Science 287: 2185-2195. - PubMed
-
- Allshire R.C., Javerzat, J.P., Redhead, N.J., and Cranston, G. 1994. Position effect variegation at fission yeast centromeres. Cell 76: 157-169. - PubMed
-
- Arn P.H., Li, X., Smith, C., Hsu, M., Schwartz, D.C., and Jabs, E.W. 1991. Analysis of DNA restriction fragments greater than 5.7 Mb in size from the centromeric region of human chromosomes. Mamm. Genome 1: 249-254. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
