Relationship between the heat tolerance of photosynthesis and the thermal stability of rubisco activase in plants from contrasting thermal environments
- PMID: 15084731
- PMCID: PMC419822
- DOI: 10.1104/pp.103.038323
Relationship between the heat tolerance of photosynthesis and the thermal stability of rubisco activase in plants from contrasting thermal environments
Abstract
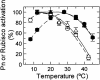
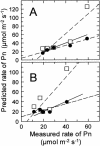
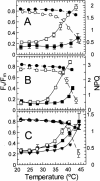
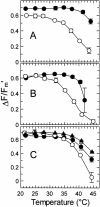
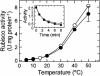
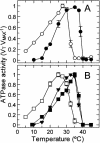
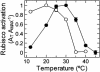
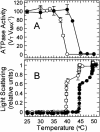
Inhibition of net photosynthesis (Pn) by moderate heat stress has been attributed to an inability of Rubisco activase to maintain Rubisco in an active form. To examine this proposal, the temperature response of Pn, Rubisco activation, chlorophyll fluorescence, and the activities of Rubisco and Rubisco activase were examined in species from contrasting environments. The temperature optimum of Rubisco activation was 10 degrees C higher in the creosote bush (Larrea tridentata) compared with the Antarctic hairgrass (Deschampsia antarctica), resembling the temperature response of Pn. Pn increased markedly with increasing internal CO(2) concentration in Antarctic hairgrass and creosote bush plants subjected to moderate heat stress even under nonphotorespiratory conditions. Nonphotochemical quenching of chlorophyll fluorescence, the effective quantum yield of photochemical energy conversion (DeltaF/F(m)') and the maximum yield of PSII (F(v)/F(m)) were more sensitive to temperature in Antarctic hairgrass and two other species endemic to cold regions (i.e. Lysipomia pumila and spinach [Spinacea oleracea]) compared with creosote bush and three species (i.e. jojoba [Simmondsia chinensis], tobacco [Nicotiana tabacum], and cotton [Gossypium hirsutum]) from warm regions. The temperature response of activity and the rate of catalytic inactivation of Rubisco from creosote bush and Antarctic hairgrass were similar, whereas the optimum for ATP hydrolysis and Rubisco activation by recombinant creosote bush, cotton, and tobacco activase was 8 degrees C to 10 degrees C higher than for Antarctic hairgrass and spinach activase. These results support a role for activase in limiting photosynthesis at high temperature.
Figures
References
-
- Alberdi M, Bravo LA, Gutiérrez A, Gidekel M, Corcuera LJ (2002) Ecophysiology of Antarctic vascular plants. Physiol Plant 115: 479–486 - PubMed
-
- Andrews TJ (1996) The bait in the Rubisco mousetrap. Nat Struct Biol 3: 3–7 - PubMed
-
- Andrews TJ, Badger MR, Lorimer GH (1975) Factors affecting interconversion between kinetic forms of ribulose diphosphate carboxylase-oxygenase from spinach. Arch Biochem Biophys 171: 93–103 - PubMed
-
- Berry J, Björkman O (1980) Photosynthetic response and adaptation to temperature in higher plants. Annu Rev Plant Physiol 31: 491–543
-
- Bilger W, Schreiber U, Lange OL (1987) Chlorophyll fluorescence as an indicator of heat induced limitation of photosynthesis in Arbutus unedo. In JD Tenhumen, FM Catarino, OL Lange, WC Oechel, eds, Plant Response to Stress. Springer-Verlag, Berlin, pp 391–399
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources

