Utilization of the plant hormone indole-3-acetic acid for growth by Pseudomonas putida strain 1290
- PMID: 15870323
- PMCID: PMC1087548
- DOI: 10.1128/AEM.71.5.2365-2371.2005
Utilization of the plant hormone indole-3-acetic acid for growth by Pseudomonas putida strain 1290
Abstract
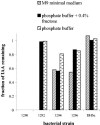
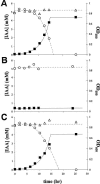
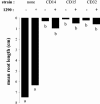
We have isolated from plant surfaces several bacteria with the ability to catabolize indole-3-acetic acid (IAA). One of them, isolate 1290, was able to utilize IAA as a sole source of carbon, nitrogen, and energy. The strain was identified by its 16S rRNA sequence as Pseudomonas putida. Activity of the enzyme catechol 1,2-dioxygenase was induced during growth on IAA, suggesting that catechol is an intermediate of the IAA catabolic pathway. This was in agreement with the observation that the oxygen uptake by IAA-grown P. putida 1290 cells was elevated in response to the addition of catechol. The inability of a catR mutant of P. putida 1290 to grow at the expense of IAA also suggests a central role for catechol as an intermediate in IAA metabolism. Besides being able to destroy IAA, strain 1290 was also capable of producing IAA in media supplemented with tryptophan. In root elongation assays, P. putida strain 1290 completely abolished the inhibitory effect of exogenous IAA on the elongation of radish roots. In fact, coinoculation of roots with P. putida 1290 and 1 mM concentration of IAA had a positive effect on root development. In coinoculation experiments on radish roots, strain 1290 was only partially able to alleviate the inhibitory effect of bacteria that in culture overproduce IAA. Our findings imply a biological role for strain 1290 as a sink or recycler of IAA in its association with plants and plant-associated bacteria.
Figures



References
-
- Bouillenne-Walrand, M., C. Leyh, and T. Gaspar. 1963. Mise en évidence d'un protecteur de l'acide beta indol-acétique dans un extrait de feuilles de Zea mays L. Bull. Soc. R. Sci. Liege 4:262-268.
-
- Bruckner, B., and D. Blechschmidt. 1991. The gibberellin fermentation. Crit. Rev. Biotechnol. 11:163-192.
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

