Alternative mutations of a positively selected residue elicit gain or loss of functionalities in enzyme evolution
- PMID: 16549767
- PMCID: PMC1458763
- DOI: 10.1073/pnas.0600849103
Alternative mutations of a positively selected residue elicit gain or loss of functionalities in enzyme evolution
Abstract
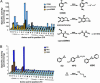
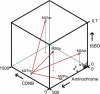
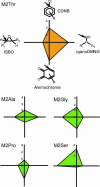
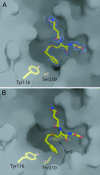
All molecular species in an organism are connected physically and functionally to other molecules. In evolving systems, it is not obvious to what extent functional properties of a protein can change to selective advantage and leave intact favorable traits previously acquired. This uncertainty has particular significance in the evolution of novel pathways for detoxication, because an organism challenged with new xenobiotics in the environment may still require biotransformation of previously encountered toxins. Positive selection has been proposed as an evolutionary mechanism for facile adaptive responses of proteins to changing conditions. Here, we show, by saturation mutagenesis, that mutations of a hypervariable residue in human glutathione transferase M2-2 can differentially change the enzyme's substrate-activity profile with alternative substrates and, furthermore, enable or disable dissimilar chemical reactions. Crystal structures demonstrate that activity with epoxides is enabled through removal of steric hindrance from a methyl group, whereas activities with an orthoquinone and a nitroso donor are maintained in the variant enzymes. Given the diversity of cellular activities in which a single protein can be engaged, the selective transmutation of functional properties has general significance in molecular evolution.
Conflict of interest statement
Conflict of interest statement: No conflicts declared.
Figures
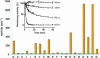
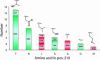
References
-
- Johnson J. M., Castle J., Garrett-Engele P., Kan Z., Loerch P. M., Armour C. D., Santos R., Schadt E. E., Stoughton R., Shoemaker D. D. Science. 2003;302:2141–2144. - PubMed
-
- Pearson W. R. Methods Enzymol. 2005;401:185–201. - PubMed
-
- Mannervik B., Board P. G., Hayes J. D., Listowsky I., Pearson W. R. Methods Enzymol. 2005;401:1–8. - PubMed
-
- Ivarsson Y., Mackey A. J., Edalat M., Pearson W. R., Mannervik B. J. Biol. Chem. 2003;278:8733–8738. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

