The plant-specific ssDNA binding protein OSB1 is involved in the stoichiometric transmission of mitochondrial DNA in Arabidopsis
- PMID: 17189341
- PMCID: PMC1785405
- DOI: 10.1105/tpc.106.042028
The plant-specific ssDNA binding protein OSB1 is involved in the stoichiometric transmission of mitochondrial DNA in Arabidopsis
Abstract
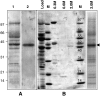
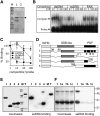
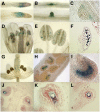
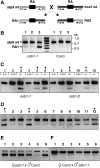
Plant mitochondrial genomes exist in a natural state of heteroplasmy, in which substoichiometric levels of alternative mitochondrial DNA (mtDNA) molecules coexist with the main genome. These subgenomes either replicate autonomously or are created by infrequent recombination events. We found that Arabidopsis thaliana OSB1 (for Organellar Single-stranded DNA Binding protein1) is required for correct stoichiometric mtDNA transmission. OSB1 is part of a family of plant-specific DNA binding proteins that are characterized by a novel motif that is required for single-stranded DNA binding. The OSB1 protein is targeted to mitochondria, and promoter-beta-glucuronidase fusion showed that the gene is expressed in budding lateral roots, mature pollen, and the embryo sac of unfertilized ovules. OSB1 T-DNA insertion mutants accumulate mtDNA homologous recombination products and develop phenotypes of leaf variegation and distortion. The mtDNA rearrangements occur in two steps: first, homozygous mutants accumulate subgenomic levels of homologous recombination products; second, in subsequent generations, one of the recombination products becomes predominant. After the second step, the process is no longer reversible by backcrossing. Thus, OSB1 participates in controlling the stoichiometry of alternative mtDNA forms generated by recombination. This regulation could take place in gametophytic tissues to ensure the transmission of a functional mitochondrial genome.
Figures




References
-
- Adams, K.L., and Daley, D. (2004). Plant mitochondrial genome evolution and gene transfer to the nucleus. In Plant Mitochondria: From Genome to Function, D.A. Day, A.H. Millar, and J. Whelan, eds (Dordrecht, The Netherlands: Kluwer Academic Publishers), pp. 107–120.
-
- Backert, S., and Börner, T. (2000). Phage T4-like intermediates of DNA replication and recombination in the mitochondria of the higher plant Chenopodium album (L.). Curr. Genet. 37 304–314. - PubMed
-
- Backert, S., Nielsen, B.L., and Börner, T. (1997). The mystery of the rings: Structure and replication of mitochondrial genomes from higher plants. Trends Plant Sci. 2 477–483.
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

