The effects of medical management on the progression of diabetic retinopathy in persons with type 2 diabetes: the Action to Control Cardiovascular Risk in Diabetes (ACCORD) Eye Study
- PMID: 25172198
- PMCID: PMC4252767
- DOI: 10.1016/j.ophtha.2014.07.019
The effects of medical management on the progression of diabetic retinopathy in persons with type 2 diabetes: the Action to Control Cardiovascular Risk in Diabetes (ACCORD) Eye Study
Abstract
Purpose: To report additional ocular outcomes of intensive treatment of hyperglycemia, blood pressure, and dyslipidemia in the Action to Control Cardiovascular Risk in Diabetes (ACCORD) study.
Design: Double 2×2 factorial, multicenter, randomized clinical trials in people with type 2 diabetes who had cardiovascular disease or cardiovascular risk factors. In the glycemia trial, targets of intensive and standard treatment were: hemoglobin A1c <6.0% and 7.0% to 7.9%, respectively, and in the blood pressure trial: systolic blood pressures of <120 and <140 mmHg, respectively. The dyslipidemia trial compared fenofibrate plus simvastatin with placebo plus simvastatin.
Participants: Of the 3472 ACCORD Eye Study participants enrolled, 2856 had 4-year data (85% of survivors).
Methods: Eye examinations and fundus photographs were taken at baseline and year 4. Photographs were graded centrally for retinopathy severity and macular edema using the Early Treatment Diabetic Retinopathy Study (ETDRS) methods.
Main outcome measures: Three or more steps of progression on the ETDRS person scale or treatment of retinopathy with photocoagulation or vitrectomy.
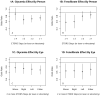
Results: As previously reported, there were significant reductions in the primary outcome in the glycemia and dyslipidemia trials, but no significant effect in the blood pressure trial. Results were similar for retinopathy progression by 1, 2, and 4 or more steps on the person scale and for ≥ 2 steps on the eye scale. In the subgroup of patients with mild retinopathy at baseline, effect estimates were large (odds ratios, ∼0.30; P < 0.001), but did not reach nominal significance for participants with no retinopathy or for those with moderate to severe retinopathy at baseline.
Conclusions: Slowing of progression of retinopathy by intensive treatment of glycemia was observed in ACCORD participants, whose average age and diabetes duration were 62 and 10 years, respectively, and who had cardiovascular disease or cardiovascular risk factors. The effect seemed stronger in patients with mild retinopathy. Similar slowing of progression was observed in patients treated with fenofibrate, with no effect observed with intensive blood pressure treatment. This is the second study to confirm the benefits of fenofibrate in reducing diabetic retinopathy progression, and fenofibrate should be considered for treatment of diabetic retinopathy.
Trial registration: ClinicalTrials.gov NCT00000620 NCT00542178.
Copyright © 2014 American Academy of Ophthalmology. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
There are no conflicts of interest from the other authors.
Figures
Comment in
-
Systemic therapies for diabetic retinopathy: the accord eye study.Ophthalmology. 2014 Dec;121(12):2295-6. doi: 10.1016/j.ophtha.2014.08.019. Epub 2014 Nov 24. Ophthalmology. 2014. PMID: 25459462 No abstract available.
References
-
- The ACCORD Study Group. Action to Control Cardiovascular Risk in Diabetes (ACCORD) Trial: design and methods. Am J Cardiol. 2007;99(Suppl):21i–33i. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- N01 HC095179/HL/NHLBI NIH HHS/United States
- N01 HC095184/HL/NHLBI NIH HHS/United States
- N01-HC-95180/HC/NHLBI NIH HHS/United States
- N01 HC095178/HL/NHLBI NIH HHS/United States
- N01 HC095181/HL/NHLBI NIH HHS/United States
- IAAY1-HC-9035/HC/NHLBI NIH HHS/United States
- N01-HC-95184/HC/NHLBI NIH HHS/United States
- IAA-Y1-HC-1010/HC/NHLBI NIH HHS/United States
- N01 HC095182/HL/NHLBI NIH HHS/United States
- N01-HC-95183/HC/NHLBI NIH HHS/United States
- N01-HC-95178/HC/NHLBI NIH HHS/United States
- N01-HC-95179/HC/NHLBI NIH HHS/United States
- N01-HC-95181/HC/NHLBI NIH HHS/United States
- ZIA EY000410/ImNIH/Intramural NIH HHS/United States
- N01 HC095180/HL/NHLBI NIH HHS/United States
- Y01 HC001010/HC/NHLBI NIH HHS/United States
- N01-HC-95182/HC/NHLBI NIH HHS/United States
- N01 HC095183/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

