Tumor growth rate as a metric of progression, response, and prognosis in pancreatic and intestinal neuroendocrine tumors
- PMID: 30642293
- PMCID: PMC6332566
- DOI: 10.1186/s12885-018-5257-x
Tumor growth rate as a metric of progression, response, and prognosis in pancreatic and intestinal neuroendocrine tumors
Abstract
Background: Lanreotide depot/autogel antitumor activity in intestinal/pancreatic neuroendocrine tumors (NETs) was demonstrated in the phase-3 CLARINET study (NCT00353496), based on significantly prolonged progression-free survival (PFS) versus placebo.
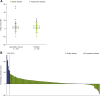
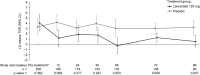
Methods: During CLARINET, patients with metastatic intestinal/pancreatic NETs received lanreotide depot/autogel 120 mg or placebo every 4 weeks for 96 weeks. Imaging data (response evaluation criteria in solid tumors [RECIST] v1.0, centrally reviewed) were re-evaluated in this post hoc analysis of tumor growth rate (TGR) in NETs. TGR (%/month) was calculated from two imaging scans during relevant periods: pre-treatment (TGR0); 12-24 weeks before randomization versus baseline; each treatment visit versus baseline (TGRTx-0); between consecutive treatment visits (TGRTx-Tx). To assess TGR as a measure of prognosis, PFS was compared for TGR0 subgroups stratified by optimum TGR0 cut-off; a multivariate analysis was conducted to identify prognostic factors for PFS.
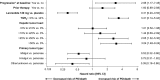
Results: TGR0 revealed tumors growing during pre-treatment (median [interquartile range] TGR0: lanreotide 2.1%/month [0.2; 6.1]; placebo 2.7%/month [0.15; 6.8]), contrary to RECIST status. TGR was significantly reduced by 12 weeks with lanreotide versus placebo (difference in least-square mean TGR0-12 of - 2.9 [- 5.1, - 0.8], p = 0.008), a difference that was maintained at most subsequent visits. TGR0 > 4%/month had greater risk of progression/death than ≤4%/month (hazard ratio 4.1; [95% CI 2.5-6.5]; p < 0.001); multivariate analysis revealed lanreotide treatment, progression at baseline, TGR0, hepatic tumor load, and primary tumor type were independently associated with PFS.
Conclusions: TGR provides valuable information on tumor activity and prognosis in patients with metastatic intestinal/pancreatic NETs, and identifies early lanreotide depot/autogel antitumor activity.
Trial registration: Retrospective registration, 18 July 2006; EudraCT: 2005-004904-35; ClinicalTrials.gov: NCT00353496 .
Keywords: Lanreotide; Neuroendocrine tumor; Prognostic factor; RECIST; Tumor growth rate.
Conflict of interest statement
Ethics approval and consent to participate
Trial documentation was approved by institutional review boards at each study site, and all patients provided written informed consent. A full list of the ethics committees and their institutions is provided in Appendix 1 of the Additional file 1.
Consent for publication
Not applicable
Competing interests
CD: Consultant/Advisor: Ipsen; MEP: Consultant/Advisor: Ipsen, Lexicon, Novartis, Pfizer; Research Support: Ipsen, Novartis; PR: Consultant/Advisor: Ipsen; Speakers bureau: Ipsen, Novartis; Research Support: Ipsen, Novartis; Employee: Ipsen (Spouse); AL: Employee: Ipsen; CM: Employee: Ipsen (at the time of work); EB: Received remuneration for services (advisory board or board of directors; corporate-sponsored research; consulting fee; research investigator; speaker) from Ipsen, Novartis, Pfizer, and Advanced Accelerator Applications; MEC: Received honoraria, consultancy and speakers’ bureau fees from Ipsen, Advanced Accelerator Applications, and Novartis.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures

References
-
- Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92(3):205–216. doi: 10.1093/jnci/92.3.205. - DOI - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

