Randomized trial of bilateral gene therapy injection for m.11778G>A MT-ND4 Leber optic neuropathy
- PMID: 36350566
- PMCID: PMC10115230
- DOI: 10.1093/brain/awac421
Randomized trial of bilateral gene therapy injection for m.11778G>A MT-ND4 Leber optic neuropathy
Abstract
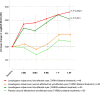
Leber hereditary optic neuropathy (LHON) is an important example of mitochondrial blindness with the m.11778G>A mutation in the MT-ND4 gene being the most common disease-causing mtDNA variant worldwide. The REFLECT phase 3 pivotal study is a randomized, double-masked, placebo-controlled trial investigating the efficacy and safety of bilateral intravitreal injection of lenadogene nolparvovec in patients with a confirmed m.11778G>A mutation, using a recombinant adeno-associated virus vector 2, serotype 2 (rAAV2/2-ND4). The first-affected eye received gene therapy; the fellow (affected/not-yet-affected) eye was randomly injected with gene therapy or placebo. The primary end point was the difference in change from baseline of best-corrected visual acuity (BCVA) in second-affected/not-yet-affected eyes treated with lenadogene nolparvovec versus placebo at 1.5 years post-treatment, expressed in logarithm of the minimal angle of resolution (LogMAR). Forty-eight patients were treated bilaterally and 50 unilaterally. At 1.5 years, the change from baseline in BCVA was not statistically different between second-affected/not-yet-affected eyes receiving lenadogene nolparvovec and placebo (primary end point). A statistically significant improvement in BCVA was reported from baseline to 1.5 years in lenadogene nolparvovec-treated eyes: -0.23 LogMAR for the first-affected eyes of bilaterally treated patients (P < 0.01); and -0.15 LogMAR for second-affected/not-yet-affected eyes of bilaterally treated patients and the first-affected eyes of unilaterally treated patients (P < 0.05). The mean improvement in BCVA from nadir to 1.5 years was -0.38 (0.052) LogMAR and -0.33 (0.052) LogMAR in first-affected and second-affected/not-yet-affected eyes treated with lenadogene nolparvovec, respectively (bilateral treatment group). A mean improvement of -0.33 (0.051) LogMAR and -0.26 (0.051) LogMAR was observed in first-affected lenadogene nolparvovec-treated eyes and second-affected/not-yet-affected placebo-treated eyes, respectively (unilateral treatment group). The proportion of patients with one or both eyes on-chart at 1.5 years was 85.4% and 72.0% for bilaterally and unilaterally treated patients, respectively. The gene therapy was well tolerated, with no systemic issues. Intraocular inflammation, which was mostly mild and well controlled with topical corticosteroids, occurred in 70.7% of lenadogene nolparvovec-treated eyes versus 10.2% of placebo-treated eyes. Among eyes treated with lenadogene nolparvovec, there was no difference in the incidence of intraocular inflammation between bilaterally and unilaterally treated patients. Overall, the REFLECT trial demonstrated an improvement of BCVA in LHON eyes carrying the m.11778G>A mtDNA mutation treated with lenadogene nolparvovec or placebo to a degree not reported in natural history studies and supports an improved benefit/risk profile for bilateral injections of lenadogene nolparvovec relative to unilateral injections.
Keywords: NADH dehydrogenase 4; leber hereditary optic neuropathy; lenadogene nolparvovec; mitochondrial DNA; recombinant adeno-associated virus vector 2.
© The Author(s) 2022. Published by Oxford University Press on behalf of the Guarantors of Brain.
Conflict of interest statement
N.J.N. is a consultant for GenSight Biologics, Santhera Pharmaceuticals, and Stealth BioTherapeutics; has received research support from GenSight Biologics and Santhera Pharmaceuticals; served on the Data Safety Monitoring Board for Quark NAION study; and is a medical legal consultant. P.Y.W.M. is a consultant for GenSight Biologics and Stealth BioTherapeutics and has received research support from GenSight Biologics and Santhera Pharmaceuticals. P.S.S. is a consultant for GenSight Biologics, Horizon Therapeutics, Invex Therapeutics, Viridian Therapeutics, and Kriya Therapeutics, and has received research support from Santhera Pharmaceuticals, GenSight Biologics, and Horizon Therapeutics; and is a medical legal consultant. M.L.M. is a consultant for GenSight Biologics and has received research support from GenSight. S.P.D. has been a fee-for-service consultant for GenSight Biologics. B.P.L. is a consultant for GenSight Biologics, 4DMT, AAVantgardeBio, Akouos, Asthena Therapeutics, Bayer, Biogen, GenSight Biologics, IVERIC Bio, MeiraGTx-Jansen Pharmaceuticals, LookoutGTx, Novartis, Opus Genetics, Oxurion, ProQR Therapeutics, Santen, Spark Therapeutics, REGENXBIO, Vedere Bio, ViGeneron, and has received research support from GenSight Biologics, MeiraGTx-Jansen Pharmaceuticals, Novartis and ProQR Therapeutics. V.C. is a consultant for GenSight Biologics, Santhera Pharmaceuticals, and Stealth BioTherapeutics, and has received research support from Santhera Pharmaceuticals and Stealth BioTherapeutics. V.B. is a consultant for GenSight Biologics. C.V.-C. is a consultant for Santhera Pharmaceuticals and GenSight Biologics. R.C.S. is a consultant for GenSight Biologics. A.A.S. is a consultant for Stealth BioTherapeutics. R.B. is a consultant for Horizon Therapeutics, Healthy Directions and Guardion Health Sciences and has received research support from Santhera Pharmaceuticals, Regenera and Quark Pharmaceuticals. E.D.C., M.R. and M.T. are employed by GenSight Biologics, the sponsor of these studies. J-.A.S. is the cofounder and shareholder of GenSight Biologics and the patent coauthor on allotopic transport.
Figures

References
-
- Carelli V, Carbonelli M, de Coo IF, et al. . International consensus statement on the clinical and therapeutic management of Leber hereditary optic neuropathy. J Neuroophthalmol. 2017;37:371–381. - PubMed
-
- Newman NJ, Yu-Wai-Man P, Biousse V, Carelli V. Understanding the molecular basis and pathogenesis of hereditary optic neuropathies: Towards improved diagnosis and management. Lancet Neurology. 2023;22:172–188. - PubMed
-
- Newman NJ, Carelli V, Taiel M, Yu-Wai-Man P. Visual outcomes in Leber hereditary optic neuropathy patients with the m.11778G>A (MTND4) mitochondrial DNA mutation. J Neuro-Ophthalmol. 2020;40:547–557. - PubMed

