Outcome after reduced chemotherapy for intermediate-risk neuroblastoma
- PMID: 20879880
- PMCID: PMC2993160
- DOI: 10.1056/NEJMoa1001527
Outcome after reduced chemotherapy for intermediate-risk neuroblastoma
Abstract
Background: The survival rate among patients with intermediate-risk neuroblastoma who receive dose-intensive chemotherapy is excellent, but the survival rate among patients who receive reduced doses of chemotherapy for shorter periods of time is not known.
Methods: We conducted a prospective, phase 3, nonrandomized trial to determine whether a 3-year estimated overall survival of more than 90% could be maintained with reductions in the duration of therapy and drug doses, using a tumor biology-based therapy assignment. Eligible patients had newly diagnosed, intermediate-risk neuroblastoma without MYCN amplification; these patients included infants (<365 days of age) who had stage 3 or 4 disease, children (≥365 days of age) who had stage 3 tumors with favorable histopathological features, and infants who had stage 4S disease with a diploid DNA index or unfavorable histopathological features. Patients who had disease with favorable histopathological features and hyperdiploidy were assigned to four cycles of chemotherapy, and those with an incomplete response or either unfavorable feature were assigned to eight cycles.
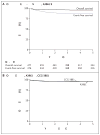
Results: Between 1997 and 2005, a total of 479 eligible patients were enrolled in this trial (270 patients with stage 3 disease, 178 with stage 4 disease, and 31 with stage 4S disease). A total of 323 patients had tumors with favorable biologic features, and 141 had tumors with unfavorable biologic features. Ploidy, but not histopathological features, was significantly predictive of the outcome. Severe adverse events without disease progression occurred in 10 patients (2.1%), including secondary leukemia (in 3 patients), death from infection (in 3 patients), and death at surgery (in 4 patients). The 3-year estimate (±SE) of overall survival for the entire group was 96±1%, with an overall survival rate of 98±1% among patients who had tumors with favorable biologic features and 93±2% among patients who had tumors with unfavorable biologic features.
Conclusions: A very high rate of survival among patients with intermediate-risk neuroblastoma was achieved with a biologically based treatment assignment involving a substantially reduced duration of chemotherapy and reduced doses of chemotherapeutic agents as compared with the regimens used in earlier trials. These data provide support for further reduction in chemotherapy with more refined risk stratification. (Funded by the National Cancer Institute; ClinicalTrials.gov number, NCT00003093.)
Figures

References
-
- Brodeur GM, Maris JM. Neuroblastoma. In: Pizzo PA, Poplack DG, editors. Principles and practice of pediatric oncology. 4. Philadelphia: Lippincott Williams & Wilkins; 2002. pp. 895–938.
-
- Seeger RC, Brodeur GM, Sather H, et al. Association of multiple copies of the N-myc oncogene with rapid progression of neuroblastoma. N Engl J Med. 1985;313:1111–6. - PubMed
-
- Brodeur GM, Seeger RC, Schwab M, Varmus HE, Bishop JM. Amplification of N-myc in untreated neuroblastomas correlates with advanced disease stage. Science. 1984;224:1121–4. - PubMed
-
- Look AT, Hayes FA, Schuster JJ, et al. Clinical relevance of tumor cell ploidy and N-myc gene amplification in childhood neuroblastoma: a Pediatric Oncology Group study. J Clin Oncol. 1991;9:581–91. - PubMed
-
- Shimada H, Umehara S, Monobe Y, et al. International neuroblastoma pathology classification for prognostic evaluation of patients with peripheral neuroblastic tumors: a report from the Children’s Cancer Group. Cancer. 2001;92:2451–61. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
