Prevalence and Clinical Implications of a β-Amyloid-Negative, Tau-Positive Cerebrospinal Fluid Biomarker Profile in Alzheimer Disease
- PMID: 37523162
- PMCID: PMC10391361
- DOI: 10.1001/jamaneurol.2023.2338
Prevalence and Clinical Implications of a β-Amyloid-Negative, Tau-Positive Cerebrospinal Fluid Biomarker Profile in Alzheimer Disease
Abstract
Importance: Knowledge is lacking on the prevalence and prognosis of individuals with a β-amyloid-negative, tau-positive (A-T+) cerebrospinal fluid (CSF) biomarker profile.
Objective: To estimate the prevalence of a CSF A-T+ biomarker profile and investigate its clinical implications.
Design, setting, and participants: This was a retrospective cohort study of the cross-sectional multicenter University of Gothenburg (UGOT) cohort (November 2019-January 2021), the longitudinal multicenter Alzheimer Disease Neuroimaging Initiative (ADNI) cohort (individuals with mild cognitive impairment [MCI] and no cognitive impairment; September 2005-May 2022), and 2 Wisconsin cohorts, Wisconsin Alzheimer Disease Research Center and Wisconsin Registry for Alzheimer Prevention (WISC; individuals without cognitive impairment; February 2007-November 2020). This was a multicenter study, with data collected from referral centers in clinical routine (UGOT) and research settings (ADNI and WISC). Eligible individuals had 1 lumbar puncture (all cohorts), 2 or more cognitive assessments (ADNI and WISC), and imaging (ADNI only) performed on 2 separate occasions. Data were analyzed on August 2022 to April 2023.
Exposures: Baseline CSF Aβ42/40 and phosphorylated tau (p-tau)181; cognitive tests (ADNI: modified preclinical Alzheimer cognitive composite [mPACC]; WISC: modified 3-test PACC [PACC-3]). Exposures in the ADNI cohort included [18F]-florbetapir amyloid positron emission tomography (PET), magnetic resonance imaging (MRI), [18F]-fluorodeoxyglucose PET (FDG-PET), and cross-sectional tau-PET (ADNI: [18F]-flortaucipir, WISC: [18F]-MK6240).
Main outcomes and measures: Primary outcomes were the prevalence of CSF AT biomarker profiles and continuous longitudinal global cognitive outcome and imaging biomarker trajectories in A-T+ vs A-T- groups. Secondary outcomes included cross-sectional tau-PET.
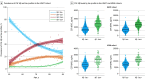
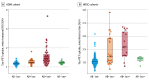
Results: A total of 7679 individuals (mean [SD] age, 71.0 [8.4] years; 4101 male [53%]) were included in the UGOT cohort, 970 individuals (mean [SD] age, 73 [7.0] years; 526 male [54%]) were included in the ADNI cohort, and 519 individuals (mean [SD] age, 60 [7.3] years; 346 female [67%]) were included in the WISC cohort. The prevalence of an A-T+ profile in the UGOT cohort was 4.1% (95% CI, 3.7%-4.6%), being less common than the other patterns. Longitudinally, no significant differences in rates of worsening were observed between A-T+ and A-T- profiles for cognition or imaging biomarkers. Cross-sectionally, A-T+ had similar tau-PET uptake to individuals with an A-T- biomarker profile.
Conclusion and relevance: Results suggest that the CSF A-T+ biomarker profile was found in approximately 5% of lumbar punctures and was not associated with a higher rate of cognitive decline or biomarker signs of disease progression compared with biomarker-negative individuals.
Conflict of interest statement
Figures


References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

