Evaluation of accuracy, exclusivity, limit-of-detection and ease-of-use of LumiraDx™: An antigen-detecting point-of-care device for SARS-CoV-2
- PMID: 34383260
- PMCID: PMC8358901
- DOI: 10.1007/s15010-021-01681-y
Evaluation of accuracy, exclusivity, limit-of-detection and ease-of-use of LumiraDx™: An antigen-detecting point-of-care device for SARS-CoV-2
Abstract
Purpose: Rapid antigen-detecting tests (Ag-RDTs) for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) can transform pandemic control. Thus far, sensitivity (≤ 85%) of lateral-flow assays has limited scale-up. Conceivably, microfluidic immunofluorescence Ag-RDTs could increase sensitivity for SARS-CoV-2 detection.
Methods: This multi-centre diagnostic accuracy study investigated performance of the microfluidic immunofluorescence LumiraDx™ assay, enrolling symptomatic and asymptomatic participants with suspected SARS-CoV-2 infection. Participants collected a supervised nasal mid-turbinate (NMT) self-swab for Ag-RDT testing, in addition to a professionally collected nasopharyngeal (NP) swab for routine testing with reverse transcriptase polymerase chain reaction (RT-PCR). Results were compared to calculate sensitivity and specificity. Sub-analyses investigated the results by viral load, symptom presence and duration. An analytical study assessed exclusivity and limit-of-detection (LOD). In addition, we evaluated ease-of-use.
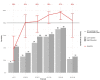
Results: The study was conducted between November 2nd 2020 and 4th of December 2020. 761 participants were enrolled, with 486 participants reporting symptoms on testing day. 120 out of 146 RT-PCR positive cases were detected positive by LumiraDx™, resulting in a sensitivity of 82.2% (95% CI 75.2-87.5%). Specificity was 99.3% (CI 98.3-99.7%). Sensitivity was increased in individuals with viral load ≥ 7 log10 SARS-CoV2 RNA copies/ml (93.8%; CI 86.2-97.3%). Testing against common respiratory commensals and pathogens showed no cross-reactivity and LOD was estimated to be 2-56 PFU/mL. The ease-of-use-assessment was favourable for lower throughput settings.
Conclusion: The LumiraDx™ assay showed excellent analytical sensitivity, exclusivity and clinical specificity with good clinical sensitivity using supervised NMT self-sampling.
Trial registration number and registration date: DRKS00021220 and 01.04.2020.
Keywords: Antigen-detecting diagnostics; Covid-19; Diagnostic accuracy; Point-of-care; SARS-CoV-2.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no conflict of interest.
Figures

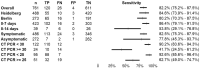

References
-
- World Health Organisation. WHO Coronavirus Disease (COVID-19) Dashboard. 2020. https://covid19.who.int/. Accessed 01 Feb 2021.
-
- World Health Organisation. Director-General’s opening remarks at the media briefing on COVID-19–16 March 2020. 2020. https://www.who.int/dg/speeches/detail/who-director-general-s-opening-re.... Accessed 12 Jan 2021.
-
- World Health Organisation. Advice on the use of point-of-care immunodiagnostic tests for COVID-19. 2020. https://www.who.int/news-room/commentaries/detail/advice-on-the-use-of-p.... Accessed 02 Jan 2021.
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

