Indirect Comparison of Lenadogene Nolparvovec Gene Therapy Versus Natural History in Patients with Leber Hereditary Optic Neuropathy Carrying the m.11778G>A MT-ND4 Mutation
- PMID: 36449262
- PMCID: PMC9834474
- DOI: 10.1007/s40123-022-00611-x
Indirect Comparison of Lenadogene Nolparvovec Gene Therapy Versus Natural History in Patients with Leber Hereditary Optic Neuropathy Carrying the m.11778G>A MT-ND4 Mutation
Abstract
Introduction: Lenadogene nolparvovec is a promising novel gene therapy for patients with Leber hereditary optic neuropathy (LHON) carrying the m.11778G>A ND4 mutation (MT-ND4). A previous pooled analysis of phase 3 studies showed an improvement in visual acuity of patients injected with lenadogene nolparvovec compared to natural history. Here, we report updated results by incorporating data from the latest phase 3 trial REFLECT in the pool, increasing the number of treated patients from 76 to 174.
Methods: The visual acuity of 174 MT-ND4-carrying patients with LHON injected in one or both eyes with lenadogene nolparvovec from four pooled phase 3 studies (REVERSE, RESCUE and their long-term extension trial RESTORE; and REFLECT trial) was compared to the spontaneous evolution of an external control group of 208 matched patients from 11 natural history studies.
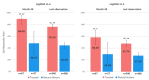
Results: Treated patients showed a clinically relevant and sustained improvement in their visual acuity when compared to natural history. Mean improvement versus natural history was - 0.30 logMAR (+ 15 ETDRS letters equivalent) at last observation (P < 0.01) with a maximal follow-up of 3.9 years after injection. Most treated eyes were on-chart as compared to less than half of natural history eyes at 48 months after vision loss (89.6% versus 48.1%; P < 0.01) and at last observation (76.1% versus 44.4%; P < 0.01). When we adjusted for covariates of interest (gender, age of onset, ethnicity, and duration of follow-up), the estimated mean gain was - 0.43 logMAR (+ 21.5 ETDRS letters equivalent) versus natural history at last observation (P < 0.0001). Treatment effect was consistent across all phase 3 clinical trials. Analyses from REFLECT suggest a larger treatment effect in patients receiving bilateral injection compared to unilateral injection.
Conclusion: The efficacy of lenadogene nolparvovec in improving visual acuity in MT-ND4 LHON was confirmed in a large cohort of patients, compared to the spontaneous natural history decline. Bilateral injection of gene therapy may offer added benefits over unilateral injection.
Trial registration numbers: NCT02652780 (REVERSE); NCT02652767 (RESCUE); NCT03406104 (RESTORE); NCT03293524 (REFLECT); NCT03295071 (REALITY).
Keywords: Gene therapy; LHON; Leber hereditary optic neuropathy; MT-ND4; Natural history; Visual acuity.
© 2022. The Author(s).
Figures