The MOnitoring Resynchronization dEvices and CARdiac patiEnts (MORE-CARE) randomized controlled trial: phase 1 results on dynamics of early intervention with remote monitoring
- PMID: 23965236
- PMCID: PMC3758044
- DOI: 10.2196/jmir.2608
The MOnitoring Resynchronization dEvices and CARdiac patiEnts (MORE-CARE) randomized controlled trial: phase 1 results on dynamics of early intervention with remote monitoring
Abstract
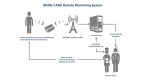
Background: Remote monitoring (RM) in patients with advanced heart failure and cardiac resynchronization therapy defibrillators (CRT-D) may reduce delays in clinical decisions by transmitting automatic alerts. However, this strategy has never been tested specifically in this patient population, with alerts for lung fluid overload, and in a European setting.
Objective: The main objective of Phase 1 (presented here) is to evaluate if RM strategy is able to reduce time from device-detected events to clinical decisions.
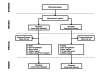
Methods: In this multicenter randomized controlled trial, patients with moderate to severe heart failure implanted with CRT-D devices were randomized to a Remote group (with remote follow-up and wireless automatic alerts) or to a Control group (with standard follow-up without alerts). The primary endpoint of Phase 1 was the delay between an alert event and clinical decisions related to the event in the first 154 enrolled patients followed for 1 year.
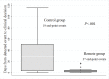
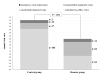
Results: The median delay from device-detected events to clinical decisions was considerably shorter in the Remote group compared to the Control group: 2 (25(th)-75(th) percentile, 1-4) days vs 29 (25(th)-75(th) percentile, 3-51) days respectively, P=.004. In-hospital visits were reduced in the Remote group (2.0 visits/patient/year vs 3.2 visits/patient/year in the Control group, 37.5% relative reduction, P<.001). Automatic alerts were successfully transmitted in 93% of events occurring outside the hospital in the Remote group. The annual rate of all-cause hospitalizations per patient did not differ between the two groups (P=.65).
Conclusions: RM in CRT-D patients with advanced heart failure allows physicians to promptly react to clinically relevant automatic alerts and significantly reduces the burden of in-hospital visits.
Trial registration: Clinicaltrials.gov NCT00885677; http://clinicaltrials.gov/show/NCT00885677 (Archived by WebCite at http://www.webcitation.org/6IkcCJ7NF).
Keywords: alerts; cardiac resynchronization therapy; heart failure; remote monitoring; telemedicine.
Conflict of interest statement
Conflicts of Interest: Arnaldo Risi, Lorenza Mangoni di S Stefano, and Xavier Navarro are employees of affiliates of Medtronic Inc. Prof. Boriani has received speaker fees from Medtronic (small amount); Dr. Ricci has received minor consultancy fees from Medtronic and Biotronik; Prof. Santini has received research grants from Medtronic, and St. Jude. Dr. Burri has received grants for research and fellowships support and speaker fees from Biotronik, Boston Scientific, Medtronic, St-Jude Medical, and Sorin. The other authors report no conflict.
Figures


References
-
- Yu CM, Wang L, Chau E, Chan RH, Kong SL, Tang MO, Christensen J, Stadler RW, Lau CP. Intrathoracic impedance monitoring in patients with heart failure: correlation with fluid status and feasibility of early warning preceding hospitalization. Circulation. 2005 Aug 9;112(6):841–8. doi: 10.1161/CIRCULATIONAHA.104.492207. http://circ.ahajournals.org/cgi/pmidlookup?view=long&pmid=16061743 - DOI - PubMed
-
- Lewalter T, Morgan J, Halimi F, Lip G, Dagres N, Blomström-Lundqvist C, Scientific Initiative Committee‚ European Heart Rhythm Association Monitoring in the management of atrial fibrillation. Europace. 2012 Apr;14(4):591–2. doi: 10.1093/europace/eus073. http://europace.oxfordjournals.org/cgi/pmidlookup?view=long&pmid=22454456 - DOI - PubMed
-
- Boriani G, Auricchio A, Klersy C, Kirchhof P, Brugada J, Morgan J, Vardas P, European Heart Rhythm Association. Eucomed Healthcare personnel resource burden related to in-clinic follow-up of cardiovascular implantable electronic devices: a European Heart Rhythm Association and Eucomed joint survey. Europace. 2011 Aug;13(8):1166–73. doi: 10.1093/europace/eur026. http://europace.oxfordjournals.org/cgi/pmidlookup?view=long&pmid=21345922 - DOI - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

