Real-World Safety and Effectiveness of Voretigene Neparvovec: Results up to 2 Years from the Prospective, Registry-Based PERCEIVE Study
- PMID: 38254722
- PMCID: PMC10813228
- DOI: 10.3390/biom14010122
Real-World Safety and Effectiveness of Voretigene Neparvovec: Results up to 2 Years from the Prospective, Registry-Based PERCEIVE Study
Abstract
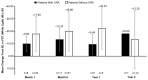
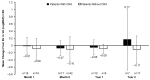
Voretigene neparvovec (VN) is the first available gene therapy for patients with biallelic RPE65-mediated inherited retinal dystrophy who have sufficient viable retinal cells. PERCEIVE is an ongoing, post-authorization, prospective, multicenter, registry-based observational study and is the largest study assessing the real-world, long-term safety and effectiveness of VN. Here, we present the outcomes of 103 patients treated with VN according to local prescribing information. The mean (SD) age was 19.5 (10.85) years, 52 (50.5%) were female, and the mean (SD) duration of the follow up was 0.8 (0.64) years (maximum: 2.3 years). Thirty-five patients (34%) experienced ocular treatment-emergent adverse events (TEAEs), most frequently related to chorioretinal atrophy (n = 13 [12.6%]). Eighteen patients (17.5%; 24 eyes [13.1%]) experienced ocular TEAEs of special interest, including intraocular inflammation and/or infection related to the procedure (n = 7). The mean (SD) changes from baseline in full-field light-sensitivity threshold testing (white light) at month 1, month 6, year 1, and year 2 were -16.59 (13.48) dB (51 eyes), -18.24 (14.62) dB (42 eyes), -15.84 (14.10) dB (10 eyes), and -13.67 (22.62) dB (13 eyes), respectively. The change in visual acuity from baseline was not clinically significant. Overall, the outcomes of the PERCEIVE study are consistent with the findings of VN pivotal clinical trials.
Trial registration: ClinicalTrials.gov NCT00516477 NCT01208389 NCT00999609.
Keywords: RPE65-associated inherited retinal dystrophies; effectiveness; real world; safety; voretigene neparvovec.
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Conflict of interest statement
Conflicts of Interest. M.D.F. reports consulting fees from Adelphi Values, Advent France Biotechnology, Adverum, Alphasights, Arctos Medical, Atheneum, Atsena, Axiom Healthcare Strategies, Bayer, Biogen, Cambridge Consultants, Decision Resources, Dialectica, Frontera Therapeutics, Hoffmann Eitle, Janssen Research and Development, MedScape, Mogrify, Navigant, Novartis, Oxford Medical Group, PeerVoice, Physicians Education Resource, Roche, RegenxBio, Sirion, Sovinnova Partners, Sparing Vision, STZeyetrial, System Analytic, Techspert, and Vindico Medical Education. He is not employed by Novartis, has no intellectual property rights, and/or financial interest in Luxturna. F.S. reports consulting fees from 3P solution, Acucela Inc., AIM group, Alia therapeutics, Allergan, AAVantgarde Bio, Bayer, Biogen, Iveric Bio, K-link SH, Kodiak, Maprovider, MeiraGTx, Novartis, Omikron, ProQR therapeutics, Santhera, SIFI, and Uvet. She is not employed by Novartis, has no intellectual property rights, and/or financial interest in Luxturna. F.G.H. reports research grants and consulting fees from Acucela, Allergan, Apellis, Bayer, Bioeq/Formycon, Roche/Genentech, Geuder, Heidelberg Engineering, ivericBio, Pixium Vision, Novartis, Zeiss; consulting fees from Alexion, Alzheon, Annexon, Astellas, Boehringer-Ingelheim, Cirrus, Grayburg Vision, LinBioscience, Stealth BioTherapeutics, Aerie, and Oxurion. R.M. and J.S. are employees of Novartis Pharma AG, Basel, Switzerland. B.C. is an employee of Novartis Pharmaceutical Corporation, USA. A.S. and D.P.S. were employees of Novartis Pharma AG when the year 2 analyses were conducted. A.S and D.P.S. are current employees of Tenpoint Therapeutics and UCB Farchim SA, respectively. C.F. is the former President of Retina International, who received unrestricted educational grants from Novartis, Roche, Biogen, Apellis, Spark Therapeutics, Inc., ProQR Therapeutics, and GenSight Biologics; reports the consulting fee from Apellis Pharmaceuticals, Inc; and is a member of advisory boards in Novartis and Hoffmann-La Roche. I.A. reports consulting fees from Novartis, ProQR Therapeutics, Roche, and 4D Molecular Therapeutics. B.P.L. reports consulting fees from 4D Molecular Therapeutics, AAVgardeBio, Akouos, Alia Therapeutics, Atsena Therapeutics, Bayer, Biogen, GenSight Biologics, Iveric Bio, Jansen Pharmaceuticals (J&J), Novartis, Opus Genetics, Oxurion, ProQR Therapeutics, RegenXBio, Santen, Spark Therapeutics, Inc, Transine Therapeutics, Vedere Bio, and ViGeneron, and travel support from GenSight Therapeutics, Iveric Bio, Novartis, and ProQR Therapeutics.
Figures



References
-
- Retinal Information Network Genes and Mapped Loci Causing Retinal Diseases. [(accessed on 16 January 2023)]. Available online: https://web.sph.uth.edu/RetNet/disease.htm.
-
- Sallum J.M.F., Kaur V.P., Shaikh J., Banhazi J., Spera C., Aouadj C., Viriato D., Fischer M.D. Epidemiology of Mutations in the 65-kDa Retinal Pigment Epithelium (RPE65) Gene-Mediated Inherited Retinal Dystrophies: A Systematic Literature Review. Adv. Ther. 2022;39:1179–1198. doi: 10.1007/s12325-021-02036-7. - DOI - PMC - PubMed
-
- Patel U., Boucher M., de Léséleuc L., Visintini S. CADTH Issues in Emerging Health Technologies. Canadian Agency for Drugs and Technologies in Health; Ottawa, ON, Canada: 2016. Voretigene Neparvovec: An Emerging Gene Therapy for the Treatment of Inherited Blindness; pp. 1–11. - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

