The CoDiNOS trial protocol: an international randomised controlled trial of intravenous sildenafil versus inhaled nitric oxide for the treatment of pulmonary hypertension in neonates with congenital diaphragmatic hernia
- PMID: 31694851
- PMCID: PMC6858099
- DOI: 10.1136/bmjopen-2019-032122
The CoDiNOS trial protocol: an international randomised controlled trial of intravenous sildenafil versus inhaled nitric oxide for the treatment of pulmonary hypertension in neonates with congenital diaphragmatic hernia
Abstract
Introduction: Congenital diaphragmatic hernia (CDH) is a developmental defect of the diaphragm that impairs normal lung development, causing pulmonary hypertension (PH). PH in CDH newborns is the main determinant for morbidity and mortality. Different therapies are still mainly based on 'trial and error'. Inhaled nitric oxide (iNO) is often the drug of first choice. However, iNO does not seem to improve mortality. Intravenous sildenafil has reduced mortality in newborns with PH without CDH, but prospective data in CDH patients are lacking.
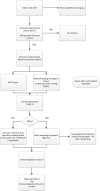
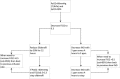
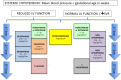
Methods and analysis: In an open label, multicentre, international randomised controlled trial in Europe, Canada and Australia, 330 newborns with CDH and PH are recruited over a 4-year period (2018-2022). Patients are randomised for intravenous sildenafil or iNO. Sildenafil is given in a loading dose of 0.4 mg/kg in 3 hours; followed by continuous infusion of 1.6 mg/kg/day, iNO is dosed at 20 ppm. Primary outcome is absence of PH on day 14 without pulmonary vasodilator therapy and/or absence of death within the first 28 days of life. Secondary outcome measures include clinical and echocardiographic markers of PH in the first year of life. We hypothesise that sildenafil gives a 25% reduction in the primary outcome from 68% to 48% on day 14, for which a sample size of 330 patients is needed. An intention-to-treat analysis will be performed. A p-value (two-sided) <0.05 is considered significant in all analyses.
Ethics and dissemination: Ethics approval has been granted by the ethics committee in Rotterdam (MEC-2017-324) and the central Committee on Research Involving Human Subjects (NL60229.078.17) in the Netherlands. The principles of the Declaration of Helsinki, the Medical Research Involving Human Subjects Act and the national rules and regulations on personal data protection will be used. Parental informed consent will be obtained.
Trial registration number: NTR6982; Pre-results.
Keywords: congenital diaphragmatic hernia; nitric oxide; pulmonary hypertension; sildenafil; therapeutics.
© Author(s) (or their employer(s)) 2019. Re-use permitted under CC BY. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures
Similar articles
-
Initial oxygenation response to inhaled nitric oxide predicts improved outcome in congenital diaphragmatic hernia.Drugs R D. 2014 Dec;14(4):215-9. doi: 10.1007/s40268-014-0063-7. Drugs R D. 2014. PMID: 25239432 Free PMC article.
-
Intravenous sildenafil in the management of pulmonary hypertension associated with congenital diaphragmatic hernia.Eur J Pediatr Surg. 2015 Apr;25(2):171-6. doi: 10.1055/s-0033-1357757. Epub 2013 Oct 25. Eur J Pediatr Surg. 2015. PMID: 24163194
-
Effect of early adjunctive use of oral sildenafil and inhaled nitric oxide on the outcome of pulmonary hypertension in newborn infants. A feasibility study.J Neonatal Perinatal Med. 2016 Sep 16;9(3):251-9. doi: 10.3233/NPM-16161. J Neonatal Perinatal Med. 2016. PMID: 27589542 Clinical Trial.
-
Management of pulmonary hypertension in infants with congenital diaphragmatic hernia.J Perinatol. 2016 Jun;36 Suppl 2:S28-31. doi: 10.1038/jp.2016.46. J Perinatol. 2016. PMID: 27225962 Review.
-
The Use of Inhaled Nitric Oxide in Congenital Diaphragmatic Hernia.Adv Neonatal Care. 2020 Dec;20(6):479-486. doi: 10.1097/ANC.0000000000000753. Adv Neonatal Care. 2020. PMID: 32384329 Review.
Cited by
-
Hemodynamic management of congenital diaphragmatic hernia: the role of targeted neonatal echocardiography.World J Pediatr Surg. 2024 May 8;7(2):e000790. doi: 10.1136/wjps-2024-000790. eCollection 2024. World J Pediatr Surg. 2024. PMID: 38737963 Free PMC article. Review.
-
Longitudinal Trajectory of Ventricular Function and Pulmonary Hypertension in Congenital Diaphragmatic Hernia.J Pediatr. 2023 Sep;260:113550. doi: 10.1016/j.jpeds.2023.113550. Epub 2023 Jun 12. J Pediatr. 2023. PMID: 37315779 Free PMC article. No abstract available.
-
Update on the use of sildenafil in neonatal pulmonary hypertension: a narrative review of the history, current administration, and future directions.Transl Pediatr. 2021 Apr;10(4):998-1007. doi: 10.21037/tp-20-277. Transl Pediatr. 2021. PMID: 34012848 Free PMC article. Review.
-
Golden hour management of infants with congenital diaphragmatic hernia: 15 year experience at a high-volume center.J Perinatol. 2025 Feb 21. doi: 10.1038/s41372-025-02226-z. Online ahead of print. J Perinatol. 2025. PMID: 39984718
-
Postnatal management of preterm infants with congenital diaphragmatic hernia.Pediatr Surg Int. 2025 Jan 16;41(1):67. doi: 10.1007/s00383-025-05964-5. Pediatr Surg Int. 2025. PMID: 39820658
References
-
- Galiè N, Hoeper MM, Humbert M, et al. . Guidelines for the diagnosis and treatment of pulmonary hypertension: the task force for the diagnosis and treatment of pulmonary hypertension of the European Society of cardiology (ESC) and the European respiratory Society (ERS), endorsed by the International Society of heart and lung transplantation (ISHLT). Eur Heart J 2009;30:2493–537. 10.1093/eurheartj/ehp297 - DOI - PubMed
-
- Russo FM, Eastwood MP, Keijzer R, et al. . Lung size and liver herniation predict need for extracorporeal membrane oxygenation but not pulmonary hypertension in isolated congenital diaphragmatic hernia: systematic review and meta-analysis. Ultrasound Obstet Gynecol 2017;49:704–13. 10.1002/uog.16000 - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
