Epilepsy in tuberous sclerosis complex: Findings from the TOSCA Study
- PMID: 30868117
- PMCID: PMC6398114
- DOI: 10.1002/epi4.12286
Epilepsy in tuberous sclerosis complex: Findings from the TOSCA Study
Abstract
Objective: To present the baseline data of the international TuberOus SClerosis registry to increase disease Awareness (TOSCA) with emphasis on the characteristics of epilepsies associated with tuberous sclerosis complex (TSC).
Methods: Retrospective and prospective patients' data on all aspects of TSC were collected from multiple countries worldwide. Epilepsy variables included seizure type, age at onset, type of treatment, and treatment outcomes and association with genotype, seizures control, and intellectual disability. As for noninterventional registries, the study protocol did not specify any particular clinical instruments, laboratory investigations, or intervention. Evaluations included those required for diagnosis and management following local best practice.
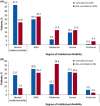
Results: Epilepsy was reported in 83.6% of patients (1852/2216) at baseline; 38.9% presented with infantile spasms and 67.5% with focal seizures. The mean age at diagnosis of infantile spasms was 0.4 year (median <1 year; range <1-30 years) and at diagnosis of focal seizures was 2.7 years (median 1 year; range <1-66 years). A total of 1469 patients (79.3%) were diagnosed with epilepsy <2 years. The rate of infantile spasms was higher in patients with a TSC 2 mutation than in patients with a TSC1 mutation (47.3% vs 23%). ɣ-aminobutyric acid (GABA)ergic drugs were the most common treatment modality for both infantile spasms (78.7%) and focal seizures (65.5%). Infantile spasms and focal seizures were controlled in 76.3% and 58.2% of patients, respectively. Control of seizures was associated with lower rates of intellectual disability in both groups.
Significance: This registry reports the largest international cohort of patients with TSC. Findings confirmed the typical onset pattern of infantile spasms and other focal seizures in the first 2 years of life, and the high rates of infantile spasms in patients with TSC2 mutation. Our results underscored the occurrence of focal seizures at all ages, including an onset that preceded emergence of infantile spasms. Seizure control was shown to be associated with lower rates of intellectual disability but did not preclude the presence of intellectual disability.
Keywords: TOSCA; epilepsy; registry; tuberous sclerosis complex.
Conflict of interest statement
6J.C.K., E.I.B., T.C., V.C., P.C., G.B.d'A., P.J.dV., J.C.F., M.F., C.F., C.H., S.J., R.N., F.O'C., J.Q., M.S., R.T., M.D., J.A.L., A.M., S.Y., M.P.B., B.Z., and A.C.J. received honoraria and travel support from Novartis. V.C. received personal fees for consulting, lecture fees, and travel from Actelion, Bayer, Biogen Idec, Boehringer Ingelheim, Gilead, GSK, MSD, Novartis, Pfizer, Roche, and Sanofi; grants from Actelion, Boehringer Ingelheim, GSK, Pfizer, and Roche; and personal fees for developing educational material from Boehringer Ingelheim and Roche. P.J.dV. has been on the study steering group of the EXIST‐1, ‐2, and ‐3 studies sponsored by Novartis, and co‐PI on 2 investigator‐initiated studies partly funded by Novartis. R.N. received grant support paid to her institution from Eisai, and lectures fees from Nutricia, Eisai, Advicenne, and GW Pharma. Y.T. received personal fees from Novartis for lecture and received a grant from the Japanese government for intractable epilepsy research. S.J. was partly financed by the EC Seventh Framework Programme (FP7/2007‐2013; EPISTOP, grant agreement no. 602391), the Polish Ministerial funds for science (years 2013‐2018) for the implementation of international cofinanced project, and the grant EPIMARKER of the Polish National Center for Research and Development No STRATEGMED3/306306/4/2016. J.C.K., P.C., C.H., J.A.L., and J.Q. received research grant from Novartis. R.M., L.D′A., and S.S. are employees of Novartis. V.S. reported no conflict of interest. This study was funded by Novartis Pharma AG. The study participants have not received funds for their participation in the study. The results presented in this manuscript have not been published previously except as a poster presentation at the Tuberous Sclerosis Complex International Congress on November 4, 2016. We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Figures
References
-
- Jeong A, Wong M. Systemic disease manifestations associated with epilepsy in tuberous sclerosis complex. Epilepsia. 2016;57:1443–9. - PubMed
-
- Krueger DA. Management of CNS‐related disease manifestations in patients with tuberous sclerosis complex. Curr Treat Options Neurol. 2013;15:618–33. - PubMed
-
- Webb DW, Fryer AE, Osborne JP. Morbidity associated with tuberous sclerosis: a population study. Dev Med Child Neurol. 1996;38:146–55. - PubMed
-
- Curatolo P, Jozwiak S, Nabbout R. Management of epilepsy associated with tuberous sclerosis complex (TSC): clinical recommendations. Eur J Paediatr Neurol. 2012;16:582–6. - PubMed