International phase III trial assessing neoadjuvant cisplatin, methotrexate, and vinblastine chemotherapy for muscle-invasive bladder cancer: long-term results of the BA06 30894 trial
- PMID: 21502557
- PMCID: PMC3107740
- DOI: 10.1200/JCO.2010.32.3139
International phase III trial assessing neoadjuvant cisplatin, methotrexate, and vinblastine chemotherapy for muscle-invasive bladder cancer: long-term results of the BA06 30894 trial
Abstract
Purpose: This article presents the long-term results of the international multicenter randomized trial that investigated the use of neoadjuvant cisplatin, methotrexate, and vinblastine (CMV) chemotherapy in patients with muscle-invasive urothelial cancer of the bladder treated by cystectomy and/or radiotherapy. Nine hundred seventy-six patients were recruited between 1989 and 1995, and median follow-up is now 8.0 years.
Patients and methods: This was a randomized phase III trial of either no neoadjuvant chemotherapy or three cycles of CMV.
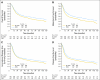
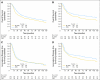
Results: The previously reported possible survival advantage of CMV is now statistically significant at the 5% level. Results show a statistically significant 16% reduction in the risk of death (hazard ratio, 0.84; 95% CI, 0.72 to 0.99; P = .037, corresponding to an increase in 10-year survival from 30% to 36%) after CMV.
Conclusion: We conclude that CMV chemotherapy improves outcome as first-line adjunctive treatment for invasive bladder cancer. Two large randomized trials (by the Medical Research Council/European Organisation for Research and Treatment of Cancer and Southwest Oncology Group) have confirmed a statistically significant and clinically relevant survival benefit, and neoadjuvant chemotherapy followed by definitive local therapy should be viewed as state of the art, as compared with cystectomy or radiotherapy alone, for deeply invasive bladder cancer.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures

Comment in
-
Kuhn's paradigms: are those closest to treating bladder cancer the last to appreciate the paradigm shift?J Clin Oncol. 2011 Jun 1;29(16):2135-7. doi: 10.1200/JCO.2010.34.0471. Epub 2011 Apr 18. J Clin Oncol. 2011. PMID: 21502548 No abstract available.
-
Bladder cancer: Time to embrace neoadjuvant chemotherapy for invasive disease.Nat Rev Urol. 2011 Jul 8;8(7):354. doi: 10.1038/nrurol.2011.95. Nat Rev Urol. 2011. PMID: 21743446 No abstract available.
-
Re: international phase III trial assessing neoadjuvant cisplatin, methotrexate, and vinblastine chemotherapy for muscle-invasive bladder cancer: long-term results of the BA06 30894 trial.J Urol. 2012 Feb;187(2):473. doi: 10.1016/j.juro.2011.10.101. Epub 2011 Dec 16. J Urol. 2012. PMID: 22237319 No abstract available.
-
Words of wisdom. Re: International phase III trial assessing neoadjuvant cisplatin, methotrexate, and vinblastin chemotherapy for muscle-invasive bladder cancer: long-term results of the BA06 30894 trial.Eur Urol. 2012 May;61(5):1063-4. doi: 10.1016/j.eururo.2012.02.012. Eur Urol. 2012. PMID: 22469414 No abstract available.
References
-
- International Collaboration of Trialists. Neoadjuvant cisplatin, methotrexate and vinblastine chemotherapy for muscle-invasive bladder cancer: A randomised controlled trial. Lancet. 1999;354:533–540. - PubMed
-
- Parmar MKB, Machin D. Chichester, United Kingdom: John Wiley & Sons; 1995. Survival Analysis: A Practical Approach.
-
- von der Maase H, Sengelov L, Roberts JT, et al. Long-term survival results of a randomized trial comparing gemcitabine plus cisplatin, with methotrexate, vinblastine, doxorubicin, plus cisplatin in patients with bladder cancer. J Clin Oncol. 2005;23:4602–4608. - PubMed
-
- Rosario DJ, Becker M, Anderson JB. The changing pattern of mortality and morbidity from radical cystectomy. BJU Int. 2000;85:427–430. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

