Relationship of Estimated GFR and Albuminuria to Concurrent Laboratory Abnormalities: An Individual Participant Data Meta-analysis in a Global Consortium
- PMID: 30348535
- PMCID: PMC6348050
- DOI: 10.1053/j.ajkd.2018.08.013
Relationship of Estimated GFR and Albuminuria to Concurrent Laboratory Abnormalities: An Individual Participant Data Meta-analysis in a Global Consortium
Abstract
Rationale & objective: Chronic kidney disease (CKD) is complicated by abnormalities that reflect disruption in filtration, tubular, and endocrine functions of the kidney. Our aim was to explore the relationship of specific laboratory result abnormalities and hypertension with the estimated glomerular filtration rate (eGFR) and albuminuria CKD staging framework.
Study design: Cross-sectional individual participant-level analyses in a global consortium.
Setting & study populations: 17 CKD and 38 general population and high-risk cohorts.
Selection criteria for studies: Cohorts in the CKD Prognosis Consortium with data for eGFR and albuminuria, as well as a measurement of hemoglobin, bicarbonate, phosphorus, parathyroid hormone, potassium, or calcium, or hypertension.
Data extraction: Data were obtained and analyzed between July 2015 and January 2018.
Analytical approach: We modeled the association of eGFR and albuminuria with hemoglobin, bicarbonate, phosphorus, parathyroid hormone, potassium, and calcium values using linear regression and with hypertension and categorical definitions of each abnormality using logistic regression. Results were pooled using random-effects meta-analyses.
Results: The CKD cohorts (n=254,666 participants) were 27% women and 10% black, with a mean age of 69 (SD, 12) years. The general population/high-risk cohorts (n=1,758,334) were 50% women and 2% black, with a mean age of 50 (16) years. There was a strong graded association between lower eGFR and all laboratory result abnormalities (ORs ranging from 3.27 [95% CI, 2.68-3.97] to 8.91 [95% CI, 7.22-10.99] comparing eGFRs of 15 to 29 with eGFRs of 45 to 59mL/min/1.73m2), whereas albuminuria had equivocal or weak associations with abnormalities (ORs ranging from 0.77 [95% CI, 0.60-0.99] to 1.92 [95% CI, 1.65-2.24] comparing urinary albumin-creatinine ratio > 300 vs < 30mg/g).
Limitations: Variations in study era, health care delivery system, typical diet, and laboratory assays.
Conclusions: Lower eGFR was strongly associated with higher odds of multiple laboratory result abnormalities. Knowledge of risk associations might help guide management in the heterogeneous group of patients with CKD.
Keywords: CKD Prognosis Consortium; CKD stage; Chronic kidney disease (CKD); albuminuria; anemia; diabetes; glomerular filtration rate (GFR); hematocrit; hemoglobin; hyperparathyroidism; hypertension; individual-level meta-analysis; kidney function; laboratory abnormality; laboratory tests; meta-analysis; serum bicarbonate; serum calcium; serum intact parathyroid hormone; serum phosphorus; serum potassium; staging system.
Copyright © 2018 National Kidney Foundation, Inc. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
The remaining authors declare that they have no relevant financial interests.
Figures
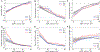
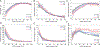

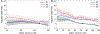
References
-
- Jha V, Garcia-Garcia G, Iseki K, et al. Chronic kidney disease: global dimension and perspectives. Lancet. 2013;382(9888):260–272. - PubMed
-
- Eckardt KU, Coresh J, Devuyst O, et al. Evolving importance of kidney disease: from subspecialty to global health burden. Lancet. 2013;382(9887):158–169. - PubMed
-
- Levin A, Tonelli M, Bonventre J, et al. Global kidney health 2017 and beyond: a roadmap for closing gaps in care, research, and policy. Lancet. 2017. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- K23 DK067303/DK/NIDDK NIH HHS/United States
- N01 HC095164/HC/NHLBI NIH HHS/United States
- P30 DK079626/DK/NIDDK NIH HHS/United States
- M01 RR000071/RR/NCRR NIH HHS/United States
- R01 HL080477/HL/NHLBI NIH HHS/United States
- N01 HC095168/HC/NHLBI NIH HHS/United States
- HHSN268201700003I/HL/NHLBI NIH HHS/United States
- N01 HC085081/HL/NHLBI NIH HHS/United States
- N01 HC095159/HC/NHLBI NIH HHS/United States
- N01 HC085080/HL/NHLBI NIH HHS/United States
- UH3 NS100605/NS/NINDS NIH HHS/United States
- M01 RR002719/RR/NCRR NIH HHS/United States
- N01 HC095167/HC/NHLBI NIH HHS/United States
- U01 HL080295/HL/NHLBI NIH HHS/United States
- UL1 TR001079/TR/NCATS NIH HHS/United States
- N01 HC085082/HL/NHLBI NIH HHS/United States
- P20 RR011104/RR/NCRR NIH HHS/United States
- U01 HL130114/HL/NHLBI NIH HHS/United States
- HHSN268200800007C/HL/NHLBI NIH HHS/United States
- N01 HC085086/HL/NHLBI NIH HHS/United States
- N01 HC085083/HL/NHLBI NIH HHS/United States
- N01 HC025195/HC/NHLBI NIH HHS/United States
- N01 HC095161/HC/NHLBI NIH HHS/United States
- N01 HC095166/HC/NHLBI NIH HHS/United States
- N01 HC095160/HC/NHLBI NIH HHS/United States
- P20 RR011145/RR/NCRR NIH HHS/United States
- R01 AG007181/AG/NIA NIH HHS/United States
- HHSN268201200036C/HL/NHLBI NIH HHS/United States
- R01 DK031801/DK/NIDDK NIH HHS/United States
- HHSN268201700001I/HL/NHLBI NIH HHS/United States
- N01 HC055222/HL/NHLBI NIH HHS/United States
- HHSN268201700004I/HL/NHLBI NIH HHS/United States
- U01 NS041588/NS/NINDS NIH HHS/United States
- M01 RR000052/RR/NCRR NIH HHS/United States
- N01 HC085079/HL/NHLBI NIH HHS/United States
- N01 HC095169/HC/NHLBI NIH HHS/United States
- HHSN268201500003I/HL/NHLBI NIH HHS/United States
- R01 DK108803/DK/NIDDK NIH HHS/United States
- M01 RR000032/RR/NCRR NIH HHS/United States
- N01 HC095165/HC/NHLBI NIH HHS/United States
- R01 HL165452/HL/NHLBI NIH HHS/United States
- UL1 TR000040/TR/NCATS NIH HHS/United States
- HHSN268201700002I/HL/NHLBI NIH HHS/United States
- HHSN268201700005I/HL/NHLBI NIH HHS/United States
- N01 HC095163/HC/NHLBI NIH HHS/United States
- R01 DK100446/DK/NIDDK NIH HHS/United States
- R01 AG023629/AG/NIA NIH HHS/United States
- N01 HC095162/HC/NHLBI NIH HHS/United States
- R01 AG028507/AG/NIA NIH HHS/United States
- M01 RR000827/RR/NCRR NIH HHS/United States
- M01 RR000080/RR/NCRR NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

