Medical management with or without interventional therapy for unruptured brain arteriovenous malformations (ARUBA): a multicentre, non-blinded, randomised trial
- PMID: 24268105
- PMCID: PMC4119885
- DOI: 10.1016/S0140-6736(13)62302-8
Medical management with or without interventional therapy for unruptured brain arteriovenous malformations (ARUBA): a multicentre, non-blinded, randomised trial
Abstract
Background: The clinical benefit of preventive eradication of unruptured brain arteriovenous malformations remains uncertain. A Randomised trial of Unruptured Brain Arteriovenous malformations (ARUBA) aims to compare the risk of death and symptomatic stroke in patients with an unruptured brain arteriovenous malformation who are allocated to either medical management alone or medical management with interventional therapy.
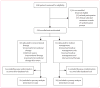
Methods: Adult patients (≥18 years) with an unruptured brain arteriovenous malformation were enrolled into this trial at 39 clinical sites in nine countries. Patients were randomised (by web-based system, in a 1:1 ratio, with random permuted block design [block size 2, 4, or 6], stratified by clinical site) to medical management with interventional therapy (ie, neurosurgery, embolisation, or stereotactic radiotherapy, alone or in combination) or medical management alone (ie, pharmacological therapy for neurological symptoms as needed). Patients, clinicians, and investigators are aware of treatment assignment. The primary outcome is time to the composite endpoint of death or symptomatic stroke; the primary analysis is by intention to treat. This trial is registered with ClinicalTrials.gov, number NCT00389181.
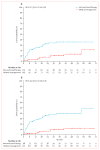
Findings: Randomisation was started on April 4, 2007, and was stopped on April 15, 2013, when a data and safety monitoring board appointed by the National Institute of Neurological Disorders and Stroke of the National Institutes of Health recommended halting randomisation because of superiority of the medical management group (log-rank Z statistic of 4·10, exceeding the prespecified stopping boundary value of 2·87). At this point, outcome data were available for 223 patients (mean follow-up 33·3 months [SD 19·7]), 114 assigned to interventional therapy and 109 to medical management. The primary endpoint had been reached by 11 (10·1%) patients in the medical management group compared with 35 (30·7%) in the interventional therapy group. The risk of death or stroke was significantly lower in the medical management group than in the interventional therapy group (hazard ratio 0·27, 95% CI 0·14-0·54). No harms were identified, other than a higher number of strokes (45 vs 12, p<0·0001) and neurological deficits unrelated to stroke (14 vs 1, p=0·0008) in patients allocated to interventional therapy compared with medical management.
Interpretation: The ARUBA trial showed that medical management alone is superior to medical management with interventional therapy for the prevention of death or stroke in patients with unruptured brain arteriovenous malformations followed up for 33 months. The trial is continuing its observational phase to establish whether the disparities will persist over an additional 5 years of follow-up.
Funding: National Institutes of Health, National Institute of Neurological Disorders and Stroke.
Copyright © 2014 Elsevier Ltd. All rights reserved.
Conflict of interest statement
We declare that we have no conflicts of interest.
Figures
Comment in
-
Management of unruptured brain arteriovenous malformations.Lancet. 2014 Feb 15;383(9917):581-3. doi: 10.1016/S0140-6736(14)60001-5. Lancet. 2014. PMID: 24529458 No abstract available.
-
Commentary: The ARUBA trial.Neurosurgery. 2014 Jul;75(1):E96-7. doi: 10.1227/NEU.0000000000000357. Neurosurgery. 2014. PMID: 24670633 No abstract available.
-
[Cerebral arteriovenous malformations: Results of the ARUBA study].Radiologe. 2014 May;54(5):422-4. doi: 10.1007/s00117-014-2675-x. Radiologe. 2014. PMID: 24692012 German. No abstract available.
-
The ARUBA study: what is the evidence?World Neurosurg. 2014 Sep-Oct;82(3-4):e576. doi: 10.1016/j.wneu.2014.04.057. Epub 2014 Apr 13. World Neurosurg. 2014. PMID: 24727184 No abstract available.
-
Management of brain arteriovenous malformations.Lancet. 2014 May 10;383(9929):1632-1633. doi: 10.1016/S0140-6736(14)60781-9. Lancet. 2014. PMID: 24814449 No abstract available.
-
Management of brain arteriovenous malformations.Lancet. 2014 May 10;383(9929):1633-1634. doi: 10.1016/S0140-6736(14)60782-0. Lancet. 2014. PMID: 24814450 No abstract available.
-
Management of brain arteriovenous malformations.Lancet. 2014 May 10;383(9929):1634. doi: 10.1016/S0140-6736(14)60783-2. Lancet. 2014. PMID: 24814451 No abstract available.
-
Management of brain arteriovenous malformations.Lancet. 2014 May 10;383(9929):1634-1635. doi: 10.1016/S0140-6736(14)60784-4. Lancet. 2014. PMID: 24814452 No abstract available.
-
Management of brain arteriovenous malformations.Lancet. 2014 May 10;383(9929):1635. doi: 10.1016/S0140-6736(14)60785-6. Lancet. 2014. PMID: 24814453 No abstract available.
-
Management of brain arteriovenous malformations--authors' reply.Lancet. 2014 May 10;383(9929):1635-1636. doi: 10.1016/S0140-6736(14)60786-8. Lancet. 2014. PMID: 24814454 No abstract available.
-
On apples, oranges, and ARUBA.Acta Neurochir (Wien). 2014 Sep;156(9):1775-9. doi: 10.1007/s00701-014-2140-7. Epub 2014 Jun 3. Acta Neurochir (Wien). 2014. PMID: 24890935 No abstract available.
-
Conservative management or intervention for unruptured brain arteriovenous malformations.World Neurosurg. 2014 Nov;82(5):e668-9. doi: 10.1016/j.wneu.2014.07.001. Epub 2014 Jul 15. World Neurosurg. 2014. PMID: 25053376 No abstract available.
-
Management of Unruptured AVMs: The Pendulum Swings.Int J Radiat Oncol Biol Phys. 2019 Nov 15;105(4):687-689. doi: 10.1016/j.ijrobp.2019.08.026. Int J Radiat Oncol Biol Phys. 2019. PMID: 31655651 No abstract available.
References
-
- Al-Shahi R, Bhattacharya JJ, Currie DG, et al. the Scottish Intracranial Vascular Malformation Study Collaborators. Prospective, population-based detection of intracranial vascular malformations in adults: the Scottish Intracranial Vascular Malformation Study (SIVMS) Stroke. 2003;34:1163–69. - PubMed
-
- Stapf C, Mast H, Sciacca RR, et al. the New York Islands AVM Study Collaborators. The New York Islands AVM Study: design, study progress, and initial results. Stroke. 2003;34:e29–33. - PubMed
-
- Ondra SL, Troupp H, George ED, Schwab K. The natural history of symptomatic arteriovenous malformations of the brain: a 24-year follow-up assessment. J Neurosurg. 1990;73:387–91. - PubMed
-
- Halim AX, Johnston SC, Singh V, et al. Longitudinal risk of intracranial hemorrhage in patients with arteriovenous malformation of the brain within a defined population. Stroke. 2004;35:1697–702. - PubMed
-
- Stapf C, Mast H, Sciacca RR, et al. Predictors of hemorrhage in patients with untreated brain arteriovenous malformation. Neurology. 2006;66:1350–55. - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

