Survey on patients' organisations' knowledge and position paper on screening for inherited neuromuscular diseases in Europe
- PMID: 33568176
- PMCID: PMC7874448
- DOI: 10.1186/s13023-020-01670-8
Survey on patients' organisations' knowledge and position paper on screening for inherited neuromuscular diseases in Europe
Abstract
Background: The development of new genetic testing methods and the approval of the first treatments raises questions regarding when and how to perform screening for inherited neuromuscular conditions. Screening directives and access to the different techniques is not uniform across Europe. The patient advisory board of the European reference network for rare neuromuscular diseases (NMD) conducted a qualitative study to understand the state of play of screening for inherited NMD in Europe and patients' needs.
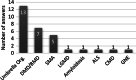
Results: We collected answers from 30 patient organisations (POs) from 18 European countries. Fifteen acknowledge the existence of pre-implantation genetic diagnosis in their country. Regarding prenatal screening, we had 25 positive answers and 5 negative ones. Twenty-four POs mentioned that newborn screening was available in their country. We had some contradictory answers from POs from the same country and in some cases; diseases said to be part of the screening programmes were not hereditary disorders. Twenty-eight organisations were in favour of screening tests. The reasons for the two negative answers were lack of reimbursement and treatment, religious beliefs and eventual insurance constrains. Most POs (21) were in favour of systematic screening with the option to opt-out. Regarding the timing for screening, "at birth", was the most consensual response. The main priority to perform screening for NMDs was early access to treatment, followed by shorter time to diagnostic, preventive care and genetic counselling.
Conclusions: This is the first study to assess knowledge and needs of POs concerning screening for NMDs. The knowledge of POs regarding screening techniques is quite uneven. This implies that, even in communities highly motivated and knowledgeable of the conditions they advocate for, there is a need for better information. Differences in the responses to the questions "how and when to screen" shows that the screening path depends on the disease and the presence of a disease modifying treatment. The unmet need for screening inherited NMDs should follow an adaptive pathway related to the fast moving medical landscape of NMDs. International coordination leading to a common policy would certainly be a precious asset tending to harmonize the situation amongst European countries.
Keywords: Neuromuscular diseases; Newborn screening; Patient organisations; Pre-conception carrier screening; Pre-implantation diagnosis; Prenatal screening.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

References
-
- Capalbo A, Romanelli V, Cimadomo D, Girardi L, Stoppa M, Dovere L, Dell'Edera D, Ubaldi FM, Rienzi L. Implementing PGD/PGD-A in IVF clinics: considerations for the best laboratory approach and management. J Assist Reprod Genet. 2016;33(10):1279–1286. doi: 10.1007/s10815-016-0768-3. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

