Implications of ACC/AHA Versus ESC/EAS LDL-C Recommendations for Residual Risk Reduction in ASCVD: A Simulation Study From DA VINCI
- PMID: 35567726
- PMCID: PMC10516778
- DOI: 10.1007/s10557-022-07343-x
Implications of ACC/AHA Versus ESC/EAS LDL-C Recommendations for Residual Risk Reduction in ASCVD: A Simulation Study From DA VINCI
Abstract
Purpose: Low-density lipoprotein cholesterol (LDL-C) recommendations differ between the 2018 American College of Cardiology/American Heart Association (ACC/AHA) and 2019 European Society of Cardiology/European Atherosclerosis Society (ESC/EAS) guidelines for patients with atherosclerotic cardiovascular disease (ASCVD) (< 70 vs. < 55 mg/dl, respectively). In the DA VINCI study, residual cardiovascular risk was predicted in ASCVD patients. The extent to which relative and absolute risk might be lowered by achieving ACC/AHA versus ESC/EAS LDL-C recommended approaches was simulated.
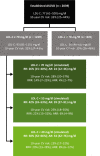
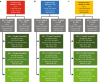
Methods: DA VINCI was a cross-sectional observational study of patients prescribed lipid-lowering therapy (LLT) across 18 European countries. Ten-year cardiovascular risk (CVR) was predicted among ASCVD patients receiving stabilized LLT. For patients with LDL-C ≥ 70 mg/dl, the absolute LDL-C reduction required to achieve an LDL-C of < 70 or < 55 mg/dl (LDL-C of 69 or 54 mg/dl, respectively) was calculated. Relative and absolute risk reductions (RRRs and ARRs) were simulated.
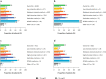
Results: Of the 2039 patients, 61% did not achieve LDL-C < 70 mg/dl. For patients with LDL-C ≥ 70 mg/dl, median (interquartile range) baseline LDL-C and 10-year CVR were 93 (81-115) mg/dl and 32% (25-43%), respectively. Median LDL-C reductions of 24 (12-46) and 39 (27-91) mg/dl were needed to achieve an LDL-C of 69 and 54 mg/dl, respectively. Attaining ACC/AHA or ESC/EAS goals resulted in simulated RRRs of 14% (7-25%) and 22% (15-32%), respectively, and ARRs of 4% (2-7%) and 6% (4-9%), respectively.
Conclusion: In ASCVD patients, achieving ESC/EAS LDL-C goals could result in a 2% additional ARR over 10 years versus the ACC/AHA approach.
Keywords: Atherosclerotic cardiovascular disease; Cardiovascular disease prevention; Cardiovascular risk; LDL-C; Lipid-lowering; Statins.
© 2022. The Author(s).
Conflict of interest statement
AJV-V acknowledges past or current participation in research grants to Imperial College London from Pfizer, Amgen, MSD, Sanofi-Aventis, Daiichi Sankyo, and Regeneron; and received personal fees for consulting from Bayer and Regeneron and honoraria for lectures from Amgen, Mylan, and Akcea; all outside the submitted work.
SB is an employee of Amgen Ltd and holds stock in Amgen.
GV is an employee of Amgen (Europe) GmbH and holds stock in Amgen.
JB has received speaker honoraria from Amgen and research grant support from AstraZeneca.
GK has no relevant disclosures.
JM has no relevant disclosures.
MB has participated in speakers bureau for Akcea, Amgen, Daiichi Sankyo, KRKA, MSD, Mylan, Polpharma, Sanofi-Aventis, Servier, and Valeant; served as a consultant to Abbott Vascular, Akcea, Amgen, Daiichi Sankyo, Esperion, Freia Pharmaceuticals, MSD, Polfarmex, Resverlogix, and Sanofi-Aventis; and received grants from Mylan, Sanofi, and Valeant.
SDS has no relevant disclosures.
DG has received honoraria for educational activities from Amgen, AstraZeneca, Berlin-Chemie, Boehringer Ingelheim, Merck, Mylan, Novartis, Novo Nordisk, Pfizer, Sanofi, and Servier.
IG-B has received personal fees and non-financial support from Akcea, Amgen and Sanofi, and personal fees from Aegerion, Amarin, Daiichi Sankyo, Novartis, and Regeneron, outside the submitted work.
GKH has received funding for clinical trial activities and/or lecture fees from Aegerion, Amgen, AstraZeneca, Eli Lilly, Genzyme, Kowa, Pfizer, Regeneron Pharmaceuticals, Roche, Sanofi, The Medicines Company, and Ionis until April 2019, and fees were paid to his institute. Since April 2019 GKH is part-time employed by Novo Nordisk.
JJJ has received research grant/support from Valeant, and has served as a consultant or speaker for ALAB Laboratories, Amgen, Bioton, Boehringer Ingelheim, Celgene, Microlife, Servier, Teva, and Valeant.
JWJ or his institution department has received research grants from, and/or was a speaker (with or without lecture fees) at meetings sponsored by Amgen, AstraZeneca, Athera, Biotronik, Boston Scientific, Dalcor, Daiichi Sankyo, Lilly, Medtronic, Merck-Schering-Plough, Pfizer, Roche, Sanofi-Aventis, The Medicine Company, the Netherlands Heart Foundation, CardioVascular Research the Netherlands (CVON), the Netherlands Heart Institute and the European Community Framework KP7 Programme.
RGK has received honoraria from, and has been a member of the speakers bureau for, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Merck, Pfizer, and Sanofi-Aventis.
SK has received grant/research support from Amgen, AstraZeneca, and Bayer; and consulting fees/honoraria from Amgen, Bayer, BMS, Boehringer Ingelheim, MSD, Mesi, Pfizer, Philips Healthcare, Sanofi, and Servier.
HKI has received honoraria for contributing to advisory boards or oral presentations from Allergan, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol Myers Squibb, MSD, Pfizer, and St Jude.
VM has no conflict of interest relevant to this publication.
LM has received fees for lectures and/or advisory work from Amarin, Amgen, Amryt, Mylan, Novartis, Sanofi-Regeneron, and Servier.
APa has received research grants and personal fees from Amgen, AstraZeneca, Bayer, Pfizer, and Sanofi-Aventis.
APe has no relevant disclosures.
PC has no relevant disclosures.
KR has received research grants for clinical trials, and consulting fees and honoraria for presentations, from Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Eli Lilly, Gilead, Merck, Mylan, Novo Nordisk, Pfizer, Sanofi, and Zentiva.
PS has received honoraria for being a speaker and advisory board member from Amgen, AstraZeneca, MSD, and Sanofi.
SR has received honoraria for being a speaker and advisory board member from Akcea, Amgen, AstraZeneca, and Sanofi; and grants from Amgen, AstraZeneca, and Sanofi.
DT has no relevant disclosures.
CV has received research grant(s)/support and honoraria from Amgen, ELPEN, MSD, Sanofi, and VIANEX.
MV has received grants, personal fees, or non-financial support from Abbott Laboratories, Amgen, AstraZeneca, Boehringer Ingelheim, KRKA, Mylan, Novartis, Novo Nordisk, Pfizer, Sanofi-Aventis, Servier, and Zentiva, outside of the submitted work.
ALC has received research grant(s)/support from Amgen, Eli Lilly, Mylan, Menarini, Sanofi, and Sanofi-Regeneron; and has served as a consultant for or received honoraria from Aegerion, Akcea, Amgen, Amryt, AstraZeneca, Daiichi Sankyo, Esperion, Genzyme, Ionis Pharmaceuticals, Kowa, Medco, Menarini, MSD, Mylan, Novartis, Recordati, Regeneron, and Sanofi.
NRP has received financial support from several pharmaceutical companies that manufacture lipid-lowering agents, for consultancy fees (Amgen and Pfizer), research projects and staff (Amgen and Pfizer), and for arranging and speaking at educational meetings (Amgen, MSD, and Pfizer). He holds no stocks and shares in any such companies. NRP is supported by the National Institute for Health Research Senior Investigator Awards, Biomedical Research Centre funding, and the British Heart Foundation Research Centre Excellence Award.
KKR reports grants from Amgen during the conduct of the study; and has received personal fees from AbbVie, Aegerion, Akcea, Algorithm, AstraZeneca, Bayer, Boehringer Ingelheim, Cerenis, Cipla, Dr Reddys, Esperion, Kowa, Lilly, Novartis, Silence Therapeutics, Takeda, The Medicines Company, and Zuellig Pharma; and grants and personal fees from Amgen, Daiichi Sankyo, MSD, Sanofi-Regeneron, and Pfizer, outside the submitted work.
Figures
References
-
- World Health Organization (2007). Prevention of cardiovascular disease: guidelines for assessment and management of total cardiovascular risk. Geneva: World Health Organization. http://www.who.int/iris/handle/10665/43685. Accessed 30 July 2021.

