ADP-ribosylating and vacuolating cytotoxin of Mycoplasma pneumoniae represents unique virulence determinant among bacterial pathogens
- PMID: 16617115
- PMCID: PMC1458948
- DOI: 10.1073/pnas.0510644103
ADP-ribosylating and vacuolating cytotoxin of Mycoplasma pneumoniae represents unique virulence determinant among bacterial pathogens
Abstract
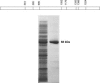
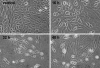
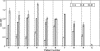
Unlike many bacterial pathogens, Mycoplasma pneumoniae is not known to produce classical toxins, and precisely how M. pneumoniae injures the respiratory epithelium has remained a mystery for >50 years. Here, we report the identification of a virulence factor (MPN372) possibly responsible for airway cellular damage and other sequelae associated with M. pneumoniae infections in humans. We show that M. pneumoniae MPN372 encodes a 68-kDa protein that possesses ADP-ribosyltransferase (ART) activity. Within its N terminus, MPN372 contains key amino acids associated with NAD binding and ADP-ribosylating activity, similar to pertussis toxin (PTX) S1 subunit (PTX-S1). Interestingly, MPN372 ADP ribosylates both identical and distinct mammalian proteins when compared with PTX-S1. Remarkably, MPN372 elicits extensive vacuolization and ultimate cell death of mammalian cells, including distinct and progressive patterns of cytopathology in tracheal rings in organ culture that had been previously ascribed to infection with WT virulent M. pneumoniae. We observed dramatic seroconversion to MPN372 in patients diagnosed with M. pneumoniae-associated pneumonia, indicating that this toxin is synthesized in vivo and possesses highly immunogenic epitopes.
Conflict of interest statement
Conflict of interest statement: No conflicts declared.
Figures

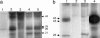

References
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

