Retrospective analysis of infliximab and adalimumab treatment in a large cohort of juvenile dermatomyositis patients
- PMID: 32293539
- PMCID: PMC7161150
- DOI: 10.1186/s13075-020-02164-5
Retrospective analysis of infliximab and adalimumab treatment in a large cohort of juvenile dermatomyositis patients
Abstract
Background: Anti-TNF treatment may be useful for the treatment of patients with refractory juvenile dermatomyositis (JDM). The aim of this study was to describe the use of infliximab and adalimumab therapy in juvenile dermatomyositis as an adjunctive treatment.
Methods: Sixty children recruited to the UK JDM Cohort and Biomarker Study that had received at least 3 months of anti-TNF treatment (infliximab or adalimumab) were studied. Childhood Myositis Assessment Scale (CMAS), Manual Muscle Testing (MMT8) and physician's global assessment (PGA) were recorded. Skin disease was assessed using the modified skin disease activity score (DAS). Data were analysed using Friedman's test for repeated measures analysis of variance.
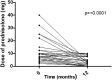
Results: Compared to baseline, there were improvements at 6 and 12 months in skin disease (χ2(2) = 15.52, p = 0.00043), global disease (χ2(2) = 8.14, p = 0.017) and muscle disease (CMAS χ2(2) = 17.02, p = 0.0002 and MMT χ2(2) = 10.56, p = 0.005) in infliximab patients. For patients who switched from infliximab to adalimumab, there was improvement in global disease activity (χ2(2) = 6.73, p = 0.03), and trends towards improvement in CMAS, MMT8 and modified DAS. The median initial prednisolone dose was 6 [0-10] mg, and final was 2.5 [0-7.5] mg (p < 0.0001). Fifty-four per cent of patients had a reduction in the number and/or size of calcinosis lesions. Twenty-five per cent switched their anti-TNF treatment from infliximab to adalimumab. 66.7%of the switches were to improve disease control, 26.7% due to adverse events and 6.6% due to patient preference. A total of 13.9 adverse reactions occurred in 100 patient-years, of which 5.7 were considered serious.
Conclusion: Reductions in muscle and skin disease, including calcinosis, were seen following treatment with infliximab and adalimumab.
Keywords: Adalimumab; Biologic therapy; Infliximab; Juvenile dermatomyositis; P rheumatology.
Conflict of interest statement
The authors declare they have no competing interests.
Figures


References
-
- Martin N, Krol P, Smith S, Murray K, Pilkington CA, Davidson JE, et al. A national registry for juvenile dermatomyositis and other paediatric idiopathic inflammatory myopathies: 10 years’ experience; the Juvenile Dermatomyositis National (UK and Ireland) Cohort Biomarker Study and Repository for Idiopathic Inflammatory Myopathies. Rheumatology (Oxford) 2011;50(1):137–145. doi: 10.1093/rheumatology/keq261. - DOI - PMC - PubMed
-
- Maini R, St Clair EW, Breedveld F, Furst D, Kalden J, Weisman M, et al. Infliximab (chimeric anti-tumour necrosis factor alpha monoclonal antibody) versus placebo in rheumatoid arthritis patients receiving concomitant methotrexate: a randomised phase III trial. ATTRACT Study Group. Lancet. 1999;354(9194):1932–1939. doi: 10.1016/S0140-6736(99)05246-0. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

