Safety and immunogenicity of concomitant administration of COVID-19 vaccines (ChAdOx1 or BNT162b2) with seasonal influenza vaccines in adults in the UK (ComFluCOV): a multicentre, randomised, controlled, phase 4 trial
- PMID: 34774197
- PMCID: PMC8585490
- DOI: 10.1016/S0140-6736(21)02329-1
Safety and immunogenicity of concomitant administration of COVID-19 vaccines (ChAdOx1 or BNT162b2) with seasonal influenza vaccines in adults in the UK (ComFluCOV): a multicentre, randomised, controlled, phase 4 trial
Abstract
Background: Concomitant administration of COVID-19 and influenza vaccines could reduce burden on health-care systems. We aimed to assess the safety of concomitant administration of ChAdOx1 or BNT162b2 plus an age-appropriate influenza vaccine.
Methods: In this multicentre, randomised, controlled, phase 4 trial, adults in receipt of a single dose of ChAdOx1 or BNT162b2 were enrolled at 12 UK sites and randomly assigned (1:1) to receive concomitant administration of either an age-appropriate influenza vaccine or placebo alongside their second dose of COVID-19 vaccine. 3 weeks later the group who received placebo received the influenza vaccine, and vice versa. Participants were followed up for 6 weeks. The influenza vaccines were three seasonal, inactivated vaccines (trivalent, MF59C adjuvanted or a cellular or recombinant quadrivalent vaccine). Participants and investigators were masked to the allocation. The primary endpoint was one or more participant-reported solicited systemic reactions in the 7 days after first trial vaccination(s), with a difference of less than 25% considered non-inferior. Analyses were done on an intention-to-treat basis. Local and unsolicited systemic reactions and humoral responses were also assessed. The trial is registered with ISRCTN, ISRCTN14391248.
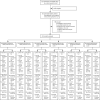
Findings: Between April 1 and June 26, 2021, 679 participants were recruited to one of six cohorts, as follows: 129 ChAdOx1 plus cellular quadrivalent influenza vaccine, 139 BNT162b2 plus cellular quadrivalent influenza vaccine, 146 ChAdOx1 plus MF59C adjuvanted, trivalent influenza vaccine, 79 BNT162b2 plus MF59C adjuvanted, trivalent influenza vaccine, 128 ChAdOx1 plus recombinant quadrivalent influenza vaccine, and 58 BNT162b2 plus recombinant quadrivalent influenza vaccine. 340 participants were assigned to concomitant administration of influenza and a second dose of COVID-19 vaccine at day 0 followed by placebo at day 21, and 339 participants were randomly assigned to concomitant administration of placebo and a second dose of COVID-19 vaccine at day 0 followed by influenza vaccine at day 21. Non-inferiority was indicated in four cohorts, as follows: ChAdOx1 plus cellular quadrivalent influenza vaccine (risk difference for influenza vaccine minus placebos -1·29%, 95% CI -14·7 to 12·1), BNT162b2 plus cellular quadrivalent influenza vaccine (6·17%, -6·27 to 18·6), BNT162b2 plus MF59C adjuvanted, trivalent influenza vaccine (-12·9%, -34·2 to 8·37), and ChAdOx1 plus recombinant quadrivalent influenza vaccine (2·53%, -13·3 to 18·3). In the other two cohorts, the upper limit of the 95% CI exceeded the 0·25 non-inferiority margin (ChAdOx1 plus MF59C adjuvanted, trivalent influenza vaccine 10·3%, -5·44 to 26·0; BNT162b2 plus recombinant quadrivalent influenza vaccine 6·75%, -11·8 to 25·3). Most systemic reactions to vaccination were mild or moderate. Rates of local and unsolicited systemic reactions were similar between the randomly assigned groups. One serious adverse event, hospitalisation with severe headache, was considered related to the trial intervention. Immune responses were not adversely affected.
Interpretation: Concomitant vaccination with ChAdOx1 or BNT162b2 plus an age-appropriate influenza vaccine raises no safety concerns and preserves antibody responses to both vaccines. Concomitant vaccination with both COVID-19 and influenza vaccines over the next immunisation season should reduce the burden on health-care services for vaccine delivery, allowing for timely vaccine administration and protection from COVID-19 and influenza for those in need.
Funding: National Institute for Health Research Policy Research Programme.
Copyright © 2021 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY-NC-ND 4.0 license. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of interests RL reports grants from the National Institute for Health Research (NIHR) during the conduct of the trial, and grants from Elizabeth Blackwell Institute, AstraZeneca, Janssen, and Valneva outside the submitted work. CAR reports grants from NIHR, during the conduct of the trial. JSN-V-T reports that he is seconded to the Department of Health and Social Care, England. AF reports grants from Pfizer during the conduct of the trial, and grants from Elizabeth Blackwell Institute, Sanofi Pasteur, VBI Vaccines, Pfizer, Janssen, GSK, MedImmune, Novavax, and Valneva outside the submitted work. AM reports grants from NIHR during the conduct of the trial, and grants from AstraZeneca, Janssen, and Valneva outside the submitted work. MDS acts on behalf of the University of Oxford as an investigator on studies funded or sponsored by vaccine manufacturers, including AstraZeneca, GSK, Pfizer, Novavax, Pfizer, Janssen, Medimmune, and MCM. All other authors declare no competing interests.
Figures



References
-
- Public Health England COVID-19 vaccine surveillance report. https://assets.publishing.service.gov.uk/government/uploads/system/uploa...
-
- Australian Government Department of Health Early advice on 2021 influenza vaccination. https://www.health.gov.au/news/early-advice-on-2021-influenza-vaccination
-
- US Centers for Disease Control and Prevention Interim clinical consideration for use of COVID-19 vaccines currently approved or authorized in the United States. https://www.cdc.gov/vaccines/covid-19/clinical-considerations/covid-19-v...
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

