HLA-A*24 is an independent predictor of 5-year progression to diabetes in autoantibody-positive first-degree relatives of type 1 diabetic patients
- PMID: 23160529
- PMCID: PMC3609594
- DOI: 10.2337/db12-0747
HLA-A*24 is an independent predictor of 5-year progression to diabetes in autoantibody-positive first-degree relatives of type 1 diabetic patients
Abstract
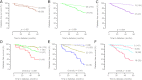
We investigated whether HLA-A*24 typing complements screening for HLA-DQ and for antibodies (Abs) against insulin, GAD, IA-2 (IA-2A), and zinc transporter-8 (ZnT8A) for prediction of rapid progression to type 1 diabetes (T1D). Persistently Ab(+) siblings/offspring (n = 288; aged 0-39 years) of T1D patients were genotyped for HLA-DQA1-DQB1 and HLA-A*24 and monitored for development of diabetes within 5 years of first Ab(+). HLA-A*24 (P = 0.009), HLA-DQ2/DQ8 (P = 0.001), and positivity for IA-2A ± ZnT8A (P < 0.001) were associated with development of T1D in multivariate analysis. The 5-year risk increased with the number of the above three markers present (n = 0: 6%; n = 1: 18%; n = 2: 46%; n = 3: 100%). Positivity for one or more markers identified a subgroup of 171 (59%) containing 88% of rapid progressors. The combined presence of HLA-A*24 and IA-2A(+) ± ZnT8A(+) defined a subgroup of 18 (6%) with an 82% diabetes risk. Among IA-2A(+) ± ZnT8A(+) relatives, identification of HLA-A*24 carriers in addition to HLA-DQ2/DQ8 carriers increased screening sensitivity for relatives at high Ab- and HLA-inferred risk (64% progression; P = 0.002). In conclusion, HLA-A*24 independently predicts rapid progression to T1D in Ab(+) relatives and complements IA-2A, ZnT8A, and HLA-DQ2/DQ8 for identifying participants in immunointervention trials.
Figures
Comment in
-
Prediction of type 1 diabetes.Diabetes. 2013 Apr;62(4):1020-1. doi: 10.2337/db12-1593. Diabetes. 2013. PMID: 23520277 Free PMC article. No abstract available.
References
-
- Todd JA. Etiology of type 1 diabetes. Immunity 2010;32:457–467 - PubMed
-
- Herold KC, Hagopian W, Auger JA, et al. Anti-CD3 monoclonal antibody in new-onset type 1 diabetes mellitus. N Engl J Med 2002;346:1692–1698 - PubMed
-
- Keymeulen B, Walter M, Mathieu C, et al. Four-year metabolic outcome of a randomised controlled CD3-antibody trial in recent-onset type 1 diabetic patients depends on their age and baseline residual beta cell mass. Diabetologia 2010;53:614–623 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

