The emergence of COVID-19 associated mucormycosis: a review of cases from 18 countries
- PMID: 35098179
- PMCID: PMC8789240
- DOI: 10.1016/S2666-5247(21)00237-8
The emergence of COVID-19 associated mucormycosis: a review of cases from 18 countries
Abstract
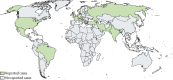
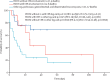
Reports of COVID-19-associated mucormycosis have been increasing in frequency since early 2021, particularly among patients with uncontrolled diabetes. Patients with diabetes and hyperglycaemia often have an inflammatory state that could be potentiated by the activation of antiviral immunity to SARS-CoV2, which might favour secondary infections. In this Review, we analysed 80 published and unpublished cases of COVID-19-associated mucormycosis. Uncontrolled diabetes, as well as systemic corticosteroid treatment, were present in most patients with COVID-19-associated mucormycosis, and rhino-orbital cerebral mucormycosis was the most frequent disease. Mortality was high at 49%, which was particularly due to patients with pulmonary or disseminated mucormycosis or cerebral involvement. Furthermore, a substantial proportion of patients who survived had life-changing morbidities (eg, loss of vision in 46% of survivors). Our Review indicates that COVID-19-associated mucormycosis is associated with high morbidity and mortality. Diagnosis of pulmonary mucormycosis is particularly challenging, and might be frequently missed in India.
© 2022 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY 4.0 license.
Conflict of interest statement
MH received research funding from Gilead, Pfizer, Astellas, Scynexis, and US National Institutes of Health. J-PG received speaker and expert advice fees from Pfizer and Gilead. NK has received research grants or honoraria as a speaker or advisor from Astellas, Gilead, MSD, and Pfizer, outside the submitted work. KL received consultancy fees from SMB Laboratories Brussels, MSD, and Gilead; travel support from Pfizer; speaker fees from FUJIFILM Wako, Pfizer, and Gilead; and a service fee from Thermo Fisher Scientific. OAC reports grants or contracts from Amplyx, Basilea, BMBF, Cidara, DZIF, EU-DG RTD (101037867), F2G, Gilead, Matinas, MedPace, MSD, Mundipharma, Octapharma, Pfizer, and Scynexis; Consulting fees from Amplyx, Biocon, Biosys, Cidara, Da Volterra, Gilead, Matinas, MedPace, Menarini, Molecular Partners, MSG-ERC, Noxxon, Octapharma, PSI, Scynexis, and Seres; honoraria for lectures from Abbott, Al-Jazeera Pharmaceuticals, Astellas, Grupo Biotoscana/United Medical/Knight, Hikma, MedScape, MedUpdate, Merck/MSD, Mylan, and Pfizer; payment for expert testimony from Cidara; participation on a data safety monitoring board or advisory board from Actelion, Allecra, Cidara, Entasis, IQVIA, Jannsen, MedPace, Paratek, PSI, and Shionogi; a patent at the German Patent and Trade Mark Office (DE 10 2021 113 0077); and other interests from DGHO, DGI, ECMM, ISHAM, MSG-ERC, and Wiley. JRP received research support from Amplyx, Appili, and Astellas; and honoraria and consulting fees from Pfizer, Appili, and Matinas. PLW has done diagnostic evaluations and received meeting sponsorship from Bruker, Dynamiker, and Launch Diagnostics; speakers fees, expert advice fees, and meeting sponsorship from Gilead; speaker and expert advice fees from F2G; speaker fees MSD and Pfizer; and is a founding member of the European Aspergillus PCR Initiative. ACh received funding support from an educational grant from Pfizer, MSD, and Gilead.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

