Systemic bud induction and retinoic acid signaling underlie whole body regeneration in the urochordate Botrylloides leachi
- PMID: 17341137
- PMCID: PMC1808485
- DOI: 10.1371/journal.pbio.0050071
Systemic bud induction and retinoic acid signaling underlie whole body regeneration in the urochordate Botrylloides leachi
Abstract
Regeneration in adult chordates is confined to a few model cases and terminates in restoration of restricted tissues and organs. Here, we study the unique phenomenon of whole body regeneration (WBR) in the colonial urochordate Botrylloides leachi in which an entire adult zooid is restored from a miniscule blood vessel fragment. In contrast to all other documented cases, regeneration is induced systemically in blood vessels. Multiple buds appear simultaneously in newly established regeneration niches within vasculature fragments, stemming from composites of pluripotent blood cells and terminating in one functional zooid. We found that retinoic acid (RA) regulates diverse developmental aspects in WBR. The homologue of the RA receptor and a retinaldehyde dehydrogenase-related gene were expressed specifically in blood cells within regeneration niches and throughout bud development. The addition of RA inhibitors as well as RNA interference knockdown experiments resulted in WBR arrest and bud malformations. The administration of all-trans RA to blood vessel fragments resulted in doubly accelerated regeneration and multibud formation, leading to restored colonies with multiple zooids. The Botrylloides system differs from known regeneration model systems by several fundamental criteria, including epimorphosis without the formation of blastema and the induction of a "multifocal regeneration niche" system. This is also to our knowledge the first documented case of WBR from circulating blood cells that restores not only the soma, but also the germ line. This unique Botrylloides WBR process could serve as a new in vivo model system for regeneration, suggesting that RA signaling may have had ancestral roles in body restoration events.
Conflict of interest statement
Figures
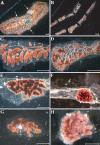
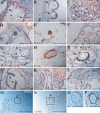



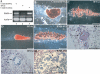

Comment in
-
From one to many and back again: a systemic signal triggers tunicate regeneration.PLoS Biol. 2007 Apr;5(4):e98. doi: 10.1371/journal.pbio.0050098. Epub 2007 Mar 6. PLoS Biol. 2007. PMID: 20076668 Free PMC article. No abstract available.
References
-
- Radtke F, Clevers H. Self-renewal and cancer of the gut: Two sides of a coin. Science. 2005;307:1904–1909. - PubMed
-
- Brockes JP, Kumar R. Appendage regeneration in adult vertebrates and implications for regenerative medicine. Science. 2005;310:1919–1923. - PubMed
-
- Reginelli AD, Wang YQ, Sasson D, Muneoka K. Digit tip regeneration correlates with regions of Msx1 (Hox 7) expression in fetal and newborn mice. Development. 1995;121:1065–1076. - PubMed
-
- Tsonis PA. Regeneration of the lens in amphibians. Results Probl Cell Differ. 2000;31:179–196. - PubMed
-
- Sanchez-Alvarado A. Regeneration in the metazoans: Why does it happen? BioEssays. 2000;22:578–590. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

