What do we know about micronutrients in critically ill patients? A narrative review
- PMID: 39555865
- PMCID: PMC11717498
- DOI: 10.1002/jpen.2700
What do we know about micronutrients in critically ill patients? A narrative review
Abstract
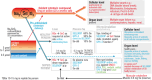
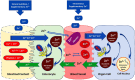
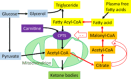
Micronutrient (MN) status alterations (both depletion and deficiency) are associated with several complications and worse outcomes in critically ill patients. On the other side of the spectrum, improving MN status has been shown to be a potential co-adjuvant therapy. This review aims to collect existing data to better guide research in the critical care setting. This narrative review was conducted by the European Society of Intensive Care Medicine Feeding, Rehabilitation, Endocrinology, and Metabolism MN group. The primary objective was to identify studies focusing on individual MNs in critically ill patients, selecting the MNs that appear to be most relevant and most frequently investigated in the last decade: A, B1, B2, B3, B6, folate, C, D, E, copper, iron, selenium, zinc, and carnitine. Given the limited number of interventional studies for most MNs, observational studies were included. For each selected MN, the review summarizes the main form and functions, special needs and risk factors, optimal treatment strategies, pharmacological dosing, and clinical implications all specific to critically ill patients. A rigorous rebalancing of research strategies and priorities is needed to improve clinical practice. An important finding is that high-dose monotherapy of MNs is not recommended. Basal daily needs must be provided, with higher doses in diseases with known higher needs, and identified deficiencies treated. Finally, the review provides a list of ongoing trials on MNs in critically ill patients and identifies a priority list of future research topics.
Keywords: critical illness; inflammation; micronutrients; nutrition; oxidative stress.
© 2024 The Author(s). Journal of Parenteral and Enteral Nutrition published by Wiley Periodicals LLC on behalf of American Society for Parenteral and Enteral Nutrition.
Conflict of interest statement
C. Stoppe receives research funding from the Department of Defense; German Research Foundation; and the companies Fresenius, BBRAUN, and Baxter. C. Stoppe has served as a consultant for Fresenius, BBRAUN, and Baxter and has received honoraria as speaker for these companies in the past. M. Casaer receives funding from the Research Foundation Flanders (FWO; grant no. 1832817N) and Onderzoeksraad, KU Leuven (grant no. C24/17/070), and from the Private Charity Organization “Help Brandwonden Kids.” J. Gunst received funding for a senior clinical investigator fellowship by the Research Foundation – Flanders. A. R. H. van Zanten reported receiving honoraria for advisory board meetings, lectures, research, and travel expenses from Abbott, AOP Pharma, Baxter, Cardinal Health, Danone‐Nutricia, DIM3, Fresenius Kabi, GE Healthcare, Inbody, Mermaid, Rousselot, and Lyric. M. M. Berger received coverage of travel expenses and lecture honoraria by Fresenius Kabi, Baxter, and Nestlé Health. A. M. E. de Man received a research grant from the Netherlands Organization of Health Research and Development and reimbursement of hotel and travel expenses as a speaker. X. Forceville started a very small start‐up in 2005 (Sérénité‐Forceville). In 2015, V. Cotereau, volunteer manager of Sérénité‐Forceville, former vice president of the French pharmacist association, filed a new patent for the treatment of sepsis entitled “Kit for treating sepsis and/or any systemic (SIRS) or damaging cellular hyper‐inflammation.” Its reference numbers are PCT number PCT/FR2016/051569, European patent application 16742342.5, and US patent application Attorney Docket number 0727‐1267. W. Manzanares received speaking honoraria from Baxter and Abbott. D. E. Bear received consulting fees from Nutricia/Danone, Avanos, Cardinal Health, and Baxter Healthcare not associated with the contents of this manuscript. The remaining authors report no conflict of interests.
Figures


References
-
- Koekkoek KWA, Berger MM. An update on essential micronutrients in critical illness. Curr Opin Crit Care. 2023;29(4):315‐329. - PubMed
-
- Berger MM, Shenkin A, Dizdar OS, et al. ESPEN practical short micronutrient guideline. Clin Nutr. 2024;43(3):825‐857. - PubMed
-
- Casaer MP, Bellomo R. Micronutrient deficiency in critical illness: an invisible foe? Intensive Care Med. 2019;45(8):1136‐1139. - PubMed
-
- Berger M, Talwar D, Shenkin A. Pitfalls in the interpretation of blood tests used to assess and monitor micronutrient nutritional status. Nutr Clin Pract. 2023;38(1):36‐69. - PubMed
-
- Vankrunkelsven W, Gunst J, Amrein K, et al. Monitoring and parenteral administration of micronutrients, phosphate and magnesium in critically ill patients: the VITA‐TRACE survey. Clin Nutr. 2021;40(2):590‐599. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

