Herpes simplex virus type 2 glycoprotein G is targeted by the sulfated oligo- and polysaccharide inhibitors of virus attachment to cells
- PMID: 17928351
- PMCID: PMC2168860
- DOI: 10.1128/JVI.01528-07
Herpes simplex virus type 2 glycoprotein G is targeted by the sulfated oligo- and polysaccharide inhibitors of virus attachment to cells
Abstract
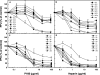
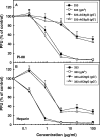
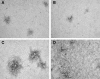
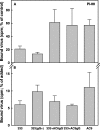
Variants of herpes simplex virus type 2 (HSV-2) generated by virus passage in GMK-AH1 cells in the presence of the sulfated oligosaccharide PI-88 were analyzed. Many of these variants were substantially resistant to PI-88 in their initial infection of cells and/or their cell-to-cell spread. The major alteration detected in all variants resistant to PI-88 in the initial infection of cells was a frameshift mutation(s) in the glycoprotein G (gG) gene that resulted in the lack of protein expression. Molecular transfer of the altered gG gene into the wild-type background confirmed that the gG-deficient recombinants were resistant to PI-88. In addition to PI-88, all gG-deficient variants of HSV-2 were resistant to the sulfated polysaccharide heparin. The gG-deficient virions were capable of attaching to cells, and this activity was relatively resistant to PI-88. In addition to having a drug-resistant phenotype, the gG-deficient variants were inefficiently released from infected cells. Purified gG bound to heparin and showed the cell-binding activity which was inhibited by PI-88. Many PI-88 variants produced syncytia in cultured cells and contained alterations in gB, including the syncytium-inducing L792P amino acid substitution. Although this phenotype can enhance the lateral spread of HSV in cells, it conferred no virus resistance to PI-88. Some PI-88 variants also contained occasional alterations in gC, gD, gE, gK, and UL24. In conclusion, we found that glycoprotein gG, a mucin-like component of the HSV-2 envelope, was targeted by sulfated oligo- and polysaccharides. This is a novel finding that suggests the involvement of HSV-2 gG in interactions with sulfated polysaccharides, including cell surface glycosaminoglycans.
Figures



References
-
- Adams, Y., C. Freeman, R. Schwartz-Albiez, V. Ferro, C. R. Parish, and K. T. Andrews. 2006. Inhibition of Plasmodium falciparum growth in vitro and adhesion to chondroitin-4-sulfate by the heparan sulfate mimetic PI-88 and other sulfated oligosaccharides. Antimicrob. Agents Chemother. 50:2850-2852. - PMC - PubMed
-
- Carlucci, M. J., L. A. Scolaro, and E. B. Damonte. 2002. Herpes simplex virus type 1 variants arising after selection with an antiviral carageenan: lack of correlation between drug susceptibility and syn phenotype. J. Med. Virol. 68:92-98. - PubMed
-
- Cheshenko, N., and B. C. Herold. 2002. Glycoprotein B plays a predominant role in mediating herpes simplex virus type 2 attachment and is required for entry and cell-to-cell spread. J. Gen. Virol. 83:2247-2255. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

