In vivo emergence of vicriviroc resistance in a human immunodeficiency virus type 1 subtype C-infected subject
- PMID: 18495779
- PMCID: PMC2519584
- DOI: 10.1128/JVI.00444-08
In vivo emergence of vicriviroc resistance in a human immunodeficiency virus type 1 subtype C-infected subject
Abstract
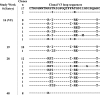
Little is known about the in vivo development of resistance to human immunodeficiency virus type 1 (HIV-1) CCR5 antagonists. We studied 29 subjects with virologic failure from a phase IIb study of the CCR5 antagonist vicriviroc (VCV) and identified one individual with HIV-1 subtype C who developed VCV resistance. Studies with chimeric envelopes demonstrated that changes within the V3 loop were sufficient to confer VCV resistance. Resistant virus showed VCV-enhanced replication, cross-resistance to another CCR5 antagonist, TAK779, and increased sensitivity to aminooxypentane-RANTES and the CCR5 monoclonal antibody HGS004. Pretreatment V3 loop sequences reemerged following VCV discontinuation, implying that VCV resistance has associated fitness costs.
Figures


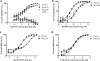
References
-
- Baba, M., O. Nishimura, N. Kanzaki, M. Okamoto, H. Sawada, Y. Iizawa, M. Shiraishi, Y. Aramaki, K. Okonogi, Y. Ogawa, K. Meguro, and M. Fujino. 1999. A small-molecule, nonpeptide CCR5 antagonist with highly potent and selective anti-HIV-1 activity. Proc. Natl. Acad. Sci. USA 965698-5703. - PMC - PubMed
-
- Gulick, R. M., Z. Su, C. Flexner, M. D. Hughes, P. R. Skolnik, T. J. Wilkin, R. Gross, A. Krambrink, E. Coakley, W. L. Greaves, A. Zolopa, R. Reichman, C. Godfrey, M. Hirsch, and D. R. Kuritzkes. 2007. Phase 2 study of the safety and efficacy of vicriviroc, a CCR5 inhibitor, in HIV-1-infected, treatment-experienced patients: AIDS clinical trials group 5211. J. Infect. Dis. 196304-312. - PubMed
-
- Kuhmann, S. E., P. Pugach, K. J. Kunstman, J. Taylor, R. L. Stanfield, A. Snyder, J. M. Strizki, J. Riley, B. M. Baroudy, I. A. Wilson, B. T. Korber, S. M. Wolinsky, and J. P. Moore. 2004. Genetic and phenotypic analyses of human immunodeficiency virus type 1 escape from a small-molecule CCR5 inhibitor. J. Virol. 782790-2807. - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

