The synaptic proteins neurexins and neuroligins are widely expressed in the vascular system and contribute to its functions
- PMID: 19926856
- PMCID: PMC2791601
- DOI: 10.1073/pnas.0809510106
The synaptic proteins neurexins and neuroligins are widely expressed in the vascular system and contribute to its functions
Abstract
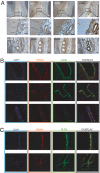
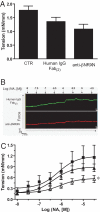
Unlike other neuronal counterparts, primary synaptic proteins are not known to be involved in vascular physiology. Here, we demonstrate that neurexins and neuroligins, which constitute large and complex families of fundamental players in synaptic activity, are produced and processed by endothelial and vascular smooth muscle cells throughout the vasculature. Moreover, they are dynamically regulated during vessel remodeling and form endogenous complexes in large vessels as well as in the brain. We used the chicken chorioallantoic membrane as a system to pursue functional studies and demonstrate that a monoclonal recombinant antibody against beta-neurexin inhibits angiogenesis, whereas exogenous neuroligin has a role in promoting angiogenesis. Finally, as an insight into the mechanism of action of beta-neurexin, we show that the anti-beta-neurexin antibody influences vessel tone in isolated chicken arteries. Our finding strongly supports the idea that even the most complex and plastic events taking place in the nervous system (i.e., synaptic activity) share molecular cues with the vascular system.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Tabuchi K, Sudhof TC. Structure and evolution of neurexin genes: Insight into the mechanism of alternative splicing. Genomics. 2002;79(6):849–859. - PubMed
-
- Rissone A, et al. Comparative genome analysis of the neurexin gene family in Danio rerio: Insights into their functions and evolution. Mol Biol Evol. 2007;24(1):236–252. - PubMed
-
- Sudhof TC. α-Latrotoxin and its receptors: Neurexins and CIRL/låtrophilins. Annu Rev Neurosci. 2001;24:933–962. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

