Inducible Siphoviruses in superficial and deep tissue isolates of Propionibacterium acnes
- PMID: 18702830
- PMCID: PMC2533672
- DOI: 10.1186/1471-2180-8-139
Inducible Siphoviruses in superficial and deep tissue isolates of Propionibacterium acnes
Abstract
Background: Propionibacterium acnes is a commensal of human skin but is also known to be involved in certain diseases, such as acne vulgaris and infections of orthopaedic implants. Treatment of these conditions is complicated by increased resistance to antibiotics and/or biofilm formation of P. acnes bacteria. P. acnes can be infected by bacteriophages, but until recently little has been known about these viruses. The aim of this study was to identify and characterize inducible phages from P. acnes on a genetic and morphological basis.
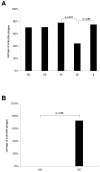
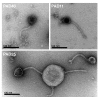
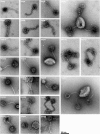
Results: More than 70% (65/92) of P. acnes isolates investigated have inducible phages, classified morphologically as Siphoviruses. The phages have a head of 55 nm in diameter and a tail of 145-155 nm in length and 9-10 nm in width. There was no difference in carriage rate of phages between P. acnes isolates from deep infections and isolates from skin. However, there was a significant lower carriage rate of phages in P. acnes biotype IB, mostly attributed to the low carriage rate of inducible phages in biotype IB isolated from deep tissue. Most phages have a strong lytic activity against all P. acnes isolates with inducible phages, but have less lytic activity against isolates that have no prophages. Phages only infected and lysed P. acnes and not other closely related propionibacteria. All phages could infect and lyse their non-induced parental host, indicating that these prophages do not confer superinfection immunity. The phages have identical protein pattern as observed on SDS-PAGE. Finally, sequencing of two phage genes encoding a putative major head protein and an amidase and showed that the phages could be divided into different groups on a genetic basis.
Conclusion: Our findings indicate that temperate phages are common in P. acnes, and that they are a genetically and functionally homogeneous group of Siphoviruses. The phages are specific for P. acnes and do not seem to confer superinfection immunity.
Figures


References
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources

