Withdrawal of antitumour necrosis factor in inflammatory bowel disease patients in remission: a randomised placebo-controlled clinical trial of GETECCU
- PMID: 39794921
- PMCID: PMC11874338
- DOI: 10.1136/gutjnl-2024-333385
Withdrawal of antitumour necrosis factor in inflammatory bowel disease patients in remission: a randomised placebo-controlled clinical trial of GETECCU
Erratum in
-
Correction: Withdrawal of antitumour necrosis factor in inflammatory bowel disease patients in remission: a randomised placebo-controlled clinical trial of GETECCU.Gut. 2025 Jul 7;74(8):e16. doi: 10.1136/gutjnl-2024-333385corr1. Gut. 2025. PMID: 40623728 No abstract available.
Abstract
Background and objectives: Primary objectives: to compare the rates of sustained clinical remission at 12 months in patients treated with antitumour necrosis factor (anti-TNF) and immunomodulators who withdraw anti-TNF treatment versus those who maintain it.
Secondary objectives: to evaluate the effect of anti-TNF withdrawal on relapse-free time, endoscopic and radiological activity, safety, quality of life and work productivity; and to identify predictive factors for relapse.
Design: Prospective, quadruple-blind, multicentre, randomised, controlled trial. Patients with ulcerative colitis or Crohn's disease in clinical remission for >6 months and absence of severe endoscopic (and radiological in Crohn's disease) lesions were randomised to maintain anti-TNF treatment (maintenance arm (MA)) or to withdraw it (withdrawal arm (WA)). All patients maintained immunomodulators. Patients were followed-up until month 12 or up to clinical relapse.
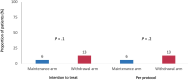
Results: One-hundred forty patients were randomised: 70 were allocated to the MA and 70 to the WA. The proportion of patients with sustained clinical remission at 12 months was similar in the MA and WA: 59/70 (84%), 95% CI=74% to 92% versus 53/70 (76%), 95% CI=64% to 85%. The proportion of patients with significant endoscopic lesions at the end of follow-up was 8.5% in the MA and 19% in the WA (p=0.1); a higher proportion of patients had faecal calprotectin >250 µg/g at the end of follow-up in the WA (p=0.01). The same percentage of patients in both groups had at least one adverse event (69%). The proportion of patients with serious adverse events was also similar in both groups (4% in MA vs 7% in WA).
Conclusion: Anti-TNF withdrawal in selected patients with IBD in clinical, endoscopic and radiological remission has no impact on sustained clinical remission at 1 year although objective markers of activity were higher in patients who withdrew treatment.
Trial registration number: https://www.clinicaltrialsregister.eu/ctr-search/search?query=2015-001410-10 https://clinicaltrials.gov/study/NCT02994836.
Keywords: INFLAMMATORY BOWEL DISEASE; INFLIXIMAB; ULCERATIVE COLITIS.
© Author(s) (or their employer(s)) 2025. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ Group.
Conflict of interest statement
Competing interests: MBdA has served as a speaker, consultant and advisory member for or has received research funding from MSD, AbbVie, Janssen, Kern Pharma, Celltrion, Takeda, GALAPAGOS, Pfizer, Sandoz, Biogen, Fresenius, Lilly, Ferring, Faes Farma, Dr. Falk Pharma, Chiesi, Gebro Pharma, Adacyte and Vifor Pharma. MCh has served as speaker, consultant or research or education funding from MSD, Abbvie, Hospira, Pfizer, Takeda, Janssen, Ferring, Shire Pharmaceuticals, Dr. Falk Pharma, Tillotts Pharma, Biogen, Gilead and Lilly. ED has served as a speaker, or has received research or education funding or advisory fees from AbbVie, Adacyte Therapeutics, Biogen, Celltrion, Galapagos, Gilead, GoodGut, Imidomics, Janssen, Kern Pharma, MSD, Pfizer, Roche, Samsung, Takeda, Tillots. JPG has served as speaker, consultant, and advisory member for or has received research funding from MSD, Abbvie, Pfizer, Kern Pharma, Biogen, Mylan, Takeda, Janssen, Roche, Sandoz, Celgene/Bristol Myers, Gilead/Galapagos, Lilly, Ferring, Faes Farma, Shire Pharmaceuticals, Dr. Falk Pharma, Tillotts Pharma, Chiesi, Casen Fleet, Gebro Pharma, Otsuka Pharmaceutical, Norgine and Vifor Pharma.Marisa Iborra reports grants and personal fees from MSD, Janssen, Abbvie, Takeda, Kern and Chiesi, during the conduct of the study.Eduardo Leo has received grants or honoraria for scientific activities, presentations or as a scientific advisor for MSD, Pfizer, Abbvie, Takeda, Janssen, Tillotts Pharma, Shire Pharmaceuticals, Ferring, Dr. Falk Pharma, Adacyte and Otsuka Pharmaceutical. PNM reports grants and personal fees from MSD, grants from Otsuka, AbbVie; personal fees from Takeda, Kern, Biogen, Ferring. JP received financial support for research from AbbVie and Pfizer; consultancy fees/honorarium from AbbVie, Arena, Athos, Atomwise, Boehringer Ingelheim, Celgene, Celsius, Celltrion, Ferring, Galapagos, Genentech/Roche, GlaxoSmithKline, Janssen, Mirum, Morphic, Pandion, Pfizer, Progenity, Prometheus, Protagonist, Revolo, Robarts, Sanofi, Takeda, Theravance and Wasserman; reports payment for lectures including service on speaker bureau from Abbott, Ferring, Janssen, Pfizer and Takeda; and reports payment for development of educational presentations from Abbott, Janssen, Pfizer Roche and Takeda. MR has served as a speaker or advisory member from TaKeda, Janssen, Galapagos, Ferring and Pfizer. MV has received educational funding from Janssen, Kern Pharma and Takeda. Rest of authors have nothing to declare.
Figures
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical