Minimal manifestation status and prednisone withdrawal in the MGTX trial
- PMID: 32611638
- PMCID: PMC7455358
- DOI: 10.1212/WNL.0000000000010031
Minimal manifestation status and prednisone withdrawal in the MGTX trial
Abstract
Objective: To examine whether sustained minimal manifestation status (MMS) with complete withdrawal of prednisone is better achieved in thymectomized patients with myasthenia gravis (MG).
Methods: This study is a post hoc analysis of data from a randomized trial of thymectomy in MG (Thymectomy Trial in Non-Thymomatous Myasthenia Gravis Patients Receiving Prednisone Therapy [MGTX]). MGTX was a multicenter, randomized, rater-blinded 3-year trial that was followed by a voluntary 2-year extension for patients with acetylcholine receptor (AChR) antibody-positive MG without thymoma. Patients were randomized 1:1 to thymectomy plus prednisone vs prednisone alone. Participants were age 18-65 years at enrollment with disease duration less than 5 years. All patients received oral prednisone titrated up to 100 mg on alternate days until they achieved MMS, which prompted a standardized prednisone taper as long as MMS was maintained. The achievement rate of sustained MMS (no symptoms of MG for 6 months) with complete withdrawal of prednisone was compared between the thymectomy plus prednisone and prednisone alone groups.
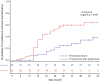
Results: Patients with MG in the thymectomy plus prednisone group achieved sustained MMS with complete withdrawal of prednisone more frequently (64% vs 38%) and quickly compared to the prednisone alone group (median time 30 months vs no median time achieved, p < 0.001) over the 5-year study period. Prednisone-associated adverse symptoms were more frequent in the prednisone alone group and distress level increased with higher doses of prednisone.
Conclusions: Thymectomy benefits patients with MG by increasing the likelihood of achieving sustained MMS with complete withdrawal of prednisone.
Clinicaltrialsgov identifier: NCT00294658.
Classification of evidence: This study provides Class II evidence that for patients with generalized MG with AChR antibody, those receiving thymectomy plus prednisone are more likely to attain sustained MMS and complete prednisone withdrawal than those on prednisone alone.
© 2020 American Academy of Neurology.
Figures
References
-
- Howard FM Jr, Duane DD, Lambert EH, Daube JR. Alternate-day prednisone: preliminary report of a double-blind controlled study. Ann NY Acad Sci 1976;274:596–607. - PubMed
-
- Mann JD, Johns TR, Campa JF, Muller WH. Long-term prednisone followed by thymectomy in myasthenia gravis. Ann NY Acad Sci 1976;274:608–622. - PubMed
-
- Pascuzzi RM, Coslett HB, Johns TR. Long-term corticosteroid treatment of myasthenia gravis: report of 116 patients. Ann Neurol 1984;15:291–298. - PubMed
-
- Sghirlanzoni A, Peluchetti D, Mantegazza R, Fiacchino F, Cornelio F. Myasthenia gravis: prolonged treatment with steroids. Neurology 1984;34:170–174. - PubMed
-
- Johns TR. Long-term corticosteroid treatment of myasthenia gravis. Ann NY Acad Sci 1987;505:568–583. - PubMed