Safety and Immunogenicity of the BNT162b2 Vaccine Coadministered with Seasonal Inactivated Influenza Vaccine in Adults
- PMID: 37698774
- PMCID: PMC10581992
- DOI: 10.1007/s40121-023-00863-5
Safety and Immunogenicity of the BNT162b2 Vaccine Coadministered with Seasonal Inactivated Influenza Vaccine in Adults
Abstract
Introduction: Vaccination is a critical tool for preventing coronavirus disease 2019 (COVID-19) and influenza illnesses. Coadministration of the COVID-19 vaccine, BNT162b2, with seasonal inactivated influenza vaccine (SIIV) can provide substantial benefits, including streamlining vaccine delivery.
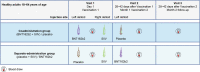
Methods: In this phase 3 study, healthy 18- to 64-year-olds who had received three previous doses of BNT162b2 were randomized (1:1) to the coadministration group (month 0, BNT162b2 + SIIV; month 1, placebo) or the separate-administration group (month 0, placebo + SIIV; month 1, BNT162b2). The primary immunogenicity objective was to demonstrate that the immune responses elicited by BNT162b2 and SIIV [measured by full-length S-binding immunoglobulin G (IgG) levels and strain-specific hemagglutination inhibition assay (HAI) titers against four influenza strains 1 month post-vaccination, respectively] when coadministered were noninferior to those elicited by either vaccine administered alone, based on a prespecified 1.5-fold noninferiority margin [lower bound 95% CI for geometric mean ratio (GMR) > 0.67]. Reactogenicity and adverse event (AE) rates were evaluated.
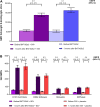
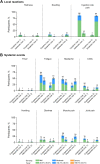
Results: Randomized participants who received study vaccination (N = 1128; coadministration group, n = 564; separate-administration group, n = 564) had a median age of 39 years. Model-adjusted GMRs for coadministration to separate administration were 0.83 (95% CI 0.77, 0.89) for full-length S-binding IgG levels and 0.89-1.00 (lower bound of all 95% CIs > 0.67) for the four influenza strain-specific HAI titers, with all endpoints achieving the prespecified noninferiority criterion. Reactogenicity events were mostly mild or moderate when BNT162b2 was coadministered with SIIV. Serious AEs were reported in < 1% of participants within 1 month after any vaccination; none were considered vaccine-related.
Conclusions: BNT162b2 coadministered with SIIV elicited immune responses that were noninferior to those elicited by BNT162b2 alone and SIIV alone, and BNT162b2 had an acceptable safety profile when coadministered with SIIV. The results of this study support the coadministration of BNT162b2 and SIIV in adults.
Trial registration: ClinicalTrials.gov registration: NCT05310084.
Keywords: BNT162b2; COVID-19; Clinical trial; Coadministration; Immunogenicity; Influenza; Safety; Seasonal inactivated influenza vaccine; Vaccination.
© 2023. The Author(s).
Conflict of interest statement
Louise Murdoch has no conflict of interest involving Pfizer or BioNTech to declare. Karen Quan, James A. Baber, Agnes W.Y. Ho, Ying Zhang, Xia Xu, Claire Lu, David Cooper, Kenneth Koury, Stephen P. Lockhart, Annaliesa S. Anderson, Kena A. Swanson, William C. Gruber, and Nicholas Kitchin are employees of Pfizer and may hold stock or stock options. Özlem Türeci and Uğur Şahin are employees of BioNTech and may hold stock or stock options.
Figures



References
-
- Surie D, DeCuir J, Zhu Y, et al. Early estimates of bivalent mRNA vaccine effectiveness in preventing COVID-19-associated hospitalization among immunocompetent adults aged ≥ 65 years—IVY network, 18 States, September 8-November 30, 2022. MMWR Morb Mortal Wkly Rep. 2022;71:1625–1630. doi: 10.15585/mmwr.mm715152e2. - DOI - PMC - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials

