Helper component proteinase of the genus Potyvirus is an interaction partner of translation initiation factors eIF(iso)4E and eIF4E and contains a 4E binding motif
- PMID: 21525344
- PMCID: PMC3126533
- DOI: 10.1128/JVI.00485-11
Helper component proteinase of the genus Potyvirus is an interaction partner of translation initiation factors eIF(iso)4E and eIF4E and contains a 4E binding motif
Abstract
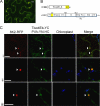
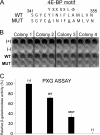
The multifunctional helper component proteinase (HCpro) of potyviruses (genus Potyvirus; Potyviridae) shows self-interaction and interacts with other potyviral and host plant proteins. Host proteins that are pivotal to potyvirus infection include the eukaryotic translation initiation factor eIF4E and the isoform eIF(iso)4E, which interact with viral genome-linked protein (VPg). Here we show that HCpro of Potato virus A (PVA) interacts with both eIF4E and eIF(iso)4E, with interactions with eIF(iso)4E being stronger, as judged by the data of a yeast two-hybrid system assay. A bimolecular fluorescence complementation assay on leaves of Nicotiana benthamiana showed that HCpro from three potyviruses (PVA, Potato virus Y, and Tobacco etch virus) interacted with the eIF(iso)4E and eIF4E of tobacco (Nicotiana tabacum); interactions with eIF(iso)4E and eIF4E of potato (Solanum tuberosum) were weaker. In PVA-infected cells, interactions between HCpro and tobacco eIF(iso)4E were confined to round structures that colocalized with 6K2-induced vesicles. Point mutations introduced to a 4E binding motif identified in the C-terminal region of HCpro debilitated interactions of HCpro with translation initiation factors and were detrimental to the virulence of PVA in plants. The 4E binding motif conserved in HCpro of potyviruses and HCpro-initiation factor interactions suggest new roles for HCpro and/or translation factors in the potyvirus infection cycle.
Figures



References
-
- Anandalakshmi R., et al. 2000. A calmodulin-related protein that suppresses posttranscriptional gene silencing in plants. Science 290:142–144 - PubMed
-
- Ayme V., et al. 2006. Different mutations in the genome-linked protein VPg of Potato virus Y confer virulence on the pvr2(3) resistance in pepper. Mol. Plant Microbe Interact. 19:557–563 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

