Unilateral nephrectomy diminishes ischemic acute kidney injury through enhanced perfusion and reduced pro-inflammatory and pro-fibrotic responses
- PMID: 29267404
- PMCID: PMC5739457
- DOI: 10.1371/journal.pone.0190009
Unilateral nephrectomy diminishes ischemic acute kidney injury through enhanced perfusion and reduced pro-inflammatory and pro-fibrotic responses
Abstract
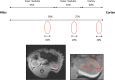
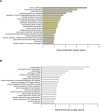
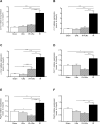
While unilateral nephrectomy (UNx) is suggested to protect against ischemia-reperfusion injury (IRI) in the remaining kidney, the mechanisms underlying this protection remain to be elucidated. In this study, functional MRI was employed in a renal IRI rat model to reveal global and regional changes in renal filtration, perfusion, oxygenation and sodium handling, and microarray and pathway analyses were conducted to identify protective molecular mechanisms. Wistar rats were randomized to either UNx or sham UNx immediately prior to 37 minutes of unilateral renal artery clamping or sham operation under sevoflurane anesthesia. MRI was performed 24 hours after reperfusion. Blood and renal tissue were harvested. RNA was isolated for microarray analysis and QPCR validation of gene expression results. The perfusion (T1 value) was significantly enhanced in the medulla of the post-ischemic kidney following UNx. UNx decreased the expression of fibrogenic genes, i.a. Col1a1, Fn1 and Tgfb1 in the post-ischemic kidney. This was associated with a marked decrease in markers of activated myofibroblasts (Acta2/α-Sma and Cdh11) and macrophages (Ccr2). This was most likely facilitated by down-regulation of Pdgfra, thus inhibiting pericyte-myofibroblast differentiation, chemokine production (Ccl2/Mcp1) and macrophage infiltration. UNx reduced ischemic histopathologic injury. UNx may exert renoprotective effects against IRI through increased perfusion in the renal medulla and alleviation of the acute pro-inflammatory and pro-fibrotic responses possibly through decreased myofibroblast activation. The identified pathways involved may serve as potential therapeutic targets and should be taken into account in experimental models of IRI.
Conflict of interest statement
Figures




References
-
- Sharfuddin AA, Weisbord SD, Palevsky PM, Molitoris BA. Acute Kidney Injury In: Skorecki K, Chertow GM, Marsden PA, Taal MW, Yu ASL, editors. Brenner and Rector's The Kidney. E-Book. 10th ed.: Elsevier Health Sciences; 2016. p. 958–1011.
-
- Nakajima T, Miyaji T, Kato A, Ikegaya N, Yamamoto T, Hishida A. Uninephrectomy reduces apoptotic cell death and enhances renal tubular cell regeneration in ischemic ARF in rats. Am J Physiol 1996. October;271(4 Pt 2):F846–53. - PubMed
-
- Fried TA, Hishida A, Barnes JL, Stein JH. Ischemic acute renal failure in the rat: protective effect of uninephrectomy. Am J Physiol 1984. October;247(4 Pt 2):F568–74. - PubMed
-
- Lin SY, Humphreys MH. Centrally administered naloxone blocks reflex natriuresis after acute unilateral nephrectomy. Am J Physiol 1985. September;249(3 Pt 2):F390–5. - PubMed
-
- Krohn AG, Peng BB, Antell HI, Stein S, Waterhouse K. Compensatory renal hypertrophy: the role of immediate vascular changes in its production. J Urol 1970. May;103(5):564–568. - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

