Loss of function of male-specific lethal 3 (Msl3) does not affect spermatogenesis in rodents
- PMID: 37847071
- PMCID: PMC11021377
- DOI: 10.1002/dvdy.669
Loss of function of male-specific lethal 3 (Msl3) does not affect spermatogenesis in rodents
Abstract
Background: Male-specific lethal 3 (Msl3) is a member of the chromatin-associated male-specific lethal MSL complex, which is responsible for the transcriptional upregulation of genes on the X chromosome in males of Drosophila. Although the dosage complex operates differently in mammals, the Msl3 gene is conserved from flies to humans. Msl3 is required for meiotic entry during Drosophila oogenesis. Recent reports indicate that also in primates, Msl3 is expressed in undifferentiated germline cells before meiotic entry. However, if Msl3 plays a role in the meiotic entry of mammals has yet to be explored.
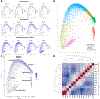
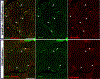
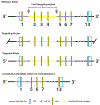
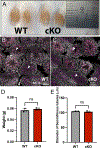
Results: To understand, if Msl3a plays a role in the meiotic entry of mammals, we used mouse spermatogenesis as a study model. Analyses of single-cell RNA-seq data revealed that, in mice, Msl3 is mostly expressed in meiotic cells. To test the role of Msl3 in meiosis, we used a male germline-specific Stra8-iCre driver and a newly generated Msl3flox conditional knock-out mouse line. Msl3 conditional loss-of-function in spermatogonia did not cause spermatogenesis defects or changes in the expression of genes related to meiosis.
Conclusions: Our data suggest that, in mice, Msl3 exhibits delayed expression compared to Drosophila and primates, and loss-of-function mutations disrupting the chromodomain of Msl3 alone do not impede meiotic entry in rodents.
Keywords: male‐specific lethal 3 (Msl3); meiosis; meiotic entry; spermatogenesis.
© 2023 American Association for Anatomy.
Conflict of interest statement
Figures

Update of
-
Loss Of Chromodomain of Male-Specific Lethal 3 (MSL3) Does Not Affect Spermatogenesis In Rodents.bioRxiv [Preprint]. 2023 Jun 27:2023.03.16.532933. doi: 10.1101/2023.03.16.532933. bioRxiv. 2023. Update in: Dev Dyn. 2024 May;253(5):453-466. doi: 10.1002/dvdy.669. PMID: 36993289 Free PMC article. Updated. Preprint.
References
-
- Clermont Y 1972. Kinetics of spermatogenesis in mammals: seminiferous epithelium cycle and spermatogonial renewal. Physiol Rev 52:198–236. - PubMed
-
- de Rooij DG. 2001. Proliferation and differentiation of spermatogonial stem cells. Reproduction 121:347–354. - PubMed
-
- Brieno-Enriquez MA, Faykoo-Martinez M, Goben M, Grenier JK, McGrath A, Prado AM, Sinopoli J, Wagner K, Walsh PT, Lopa SH, Laird DJ, Cohen PE, Wilson MD, Holmes MM, Place NJ. 2023. Postnatal oogenesis leads to an exceptionally large ovarian reserve in naked mole-rats. Nat Commun 14:670. - PMC - PubMed
-
- de Rooij DG. 2017. The nature and dynamics of spermatogonial stem cells. Development 144:3022–3030. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

