HIF-alpha effects on c-Myc distinguish two subtypes of sporadic VHL-deficient clear cell renal carcinoma
- PMID: 19061835
- PMCID: PMC2621440
- DOI: 10.1016/j.ccr.2008.10.016
HIF-alpha effects on c-Myc distinguish two subtypes of sporadic VHL-deficient clear cell renal carcinoma
Abstract
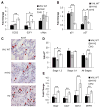
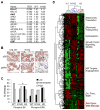
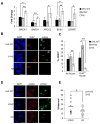
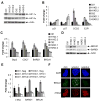
von Hippel-Lindau (VHL) tumor suppressor loss results in hypoxia-inducible factor alpha (HIF-alpha) stabilization and occurs in 70% of sporadic clear cell renal carcinomas (ccRCCs). To determine whether opposing influences of HIF-1alpha and HIF-2alpha on c-Myc activity regulate human ccRCC progression, we analyzed VHL genotype and HIF-alpha expression in 160 primary tumors, which segregated into three groups with distinct molecular characteristics. Interestingly, ccRCCs with intact VHL, as well as pVHL-deficient HIF-1alpha/HIF-2alpha-expressing ccRCCs, exhibited enhanced Akt/mTOR and ERK/MAPK signaling. In contrast, pVHL-deficient ccRCCs expressing only HIF-2alpha displayed elevated c-Myc activity, resulting in enhanced proliferation and resistance to replication stress. These reproducible distinctions in ccRCC behavior delineate HIF-alpha effects on c-Myc in vivo and suggest molecular criteria for selecting targeted therapies.
Figures


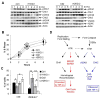
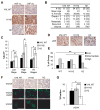
Comment in
-
Kidney cancer: now available in a new flavor.Cancer Cell. 2008 Dec 9;14(6):423-4. doi: 10.1016/j.ccr.2008.11.005. Cancer Cell. 2008. PMID: 19061830
References
-
- Banks RE, Tirukonda P, Taylor C, Hornigold N, Astuti D, Cohen D, Maher ER, Stanley AJ, Harnden P, Joyce A, et al. Genetic and epigenetic analysis of von Hippel-Lindau (VHL) gene alterations and relationship with clinical variables in sporadic renal cancer. Cancer Res. 2006;66:2000–2011. - PubMed
-
- Bartkova J, Horejsi Z, Koed K, Kramer A, Tort F, Zieger K, Guldberg P, Sehested M, Nesland JM, Lukas C, et al. DNA damage response as a candidate anti-cancer barrier in early human tumorigenesis. Nature. 2005;434:864–870. - PubMed
-
- Bartkova J, Horejsi Z, Sehested M, Nesland JM, Rajpert-De Meyts E, Skakkebaek NE, Stucki M, Jackson S, Lukas J, Bartek J. DNA damage response mediators MDC1 and 53BP1: constitutive activation and aberrant loss in breast and lung cancer, but not in testicular germ cell tumours. Oncogene. 2007;26:7414–7422. - PubMed
-
- Bartkova J, Rezaei N, Liontos M, Karakaidos P, Kletsas D, Issaeva N, Vassiliou LV, Kolettas E, Niforou K, Zoumpourlis VC, et al. Oncogene-induced senescence is part of the tumorigenesis barrier imposed by DNA damage checkpoints. Nature. 2006;444:633–637. - PubMed
-
- Bindra RS, Gibson SL, Meng A, Westermark U, Jasin M, Pierce AJ, Bristow RG, Classon MK, Glazer PM. Hypoxia-induced down-regulation of BRCA1 expression by E2Fs. Cancer Res. 2005;65:11597–11604. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

