Inducible and reversible inhibition of miRNA-mediated gene repression in vivo
- PMID: 34463618
- PMCID: PMC8476124
- DOI: 10.7554/eLife.70948
Inducible and reversible inhibition of miRNA-mediated gene repression in vivo
Abstract
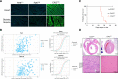
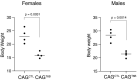
Although virtually all gene networks are predicted to be controlled by miRNAs, the contribution of this important layer of gene regulation to tissue homeostasis in adult animals remains unclear. Gain and loss-of-function experiments have provided key insights into the specific function of individual miRNAs, but effective genetic tools to study the functional consequences of global inhibition of miRNA activity in vivo are lacking. Here we report the generation and characterization of a genetically engineered mouse strain in which miRNA-mediated gene repression can be reversibly inhibited without affecting miRNA biogenesis or abundance. We demonstrate the usefulness of this strategy by investigating the consequences of acute inhibition of miRNA function in adult animals. We find that different tissues and organs respond differently to global loss of miRNA function. While miRNA-mediated gene repression is essential for the homeostasis of the heart and the skeletal muscle, it is largely dispensable in the majority of other organs. Even in tissues where it is not required for homeostasis, such as the intestine and hematopoietic system, miRNA activity can become essential during regeneration following acute injury. These data support a model where many metazoan tissues primarily rely on miRNA function to respond to potentially pathogenic events.
Keywords: RISC; T6B; TNRC6; argonaute; cell biology; miRISC; microRNA; mouse; regenerative medicine; stem cells.
© 2021, La Rocca et al.
Conflict of interest statement
GL, BK, BS, XL, MZ, KA, PO, CM, KC, VC, YM, VA, DB, JV, TT, GM, TL, KH, AV No competing interests declared, CT is a founder of Agios Pharmaceuticals and a member of its scientific advisory board. He is also a former member of the Board of Directors and stockholder of Merck and Charles River Laboratories. He is a named inventor on patents related to cellular metabolism. Potentially relevant patents on which he is a named inventor include the following: (i) L-2-hydroxyglutarate and stress induced metabolism (United States Patent #10,450,596). (ii) Single diastereomers of 4-fluoroglutamine and methods of their preparation and use (United States Patent #8,747,809). A complete list of patents can be found at the following link: https://tinyurl.com/y35qvajq
Figures















References
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

