The protein disulfide isomerase AGR2 is essential for production of intestinal mucus
- PMID: 19359471
- PMCID: PMC2678445
- DOI: 10.1073/pnas.0808722106
The protein disulfide isomerase AGR2 is essential for production of intestinal mucus
Abstract
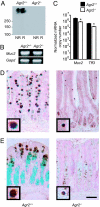
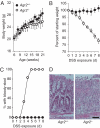
Protein disulfide isomerases (PDIs) aid protein folding and assembly by catalyzing formation and shuffling of cysteine disulfide bonds in the endoplasmic reticulum (ER). Many members of the PDI family are expressed in mammals, but the roles of specific PDIs in vivo are poorly understood. A recent homology-based search for additional PDI family members identified anterior gradient homolog 2 (AGR2), a protein originally presumed to be secreted by intestinal epithelial cells. Here, we show that AGR2 is present within the ER of intestinal secretory epithelial cells and is essential for in vivo production of the intestinal mucin MUC2, a large, cysteine-rich glycoprotein that forms the protective mucus gel lining the intestine. A cysteine residue within the AGR2 thioredoxin-like domain forms mixed disulfide bonds with MUC2, indicating a direct role for AGR2 in mucin processing. Mice lacking AGR2 were viable but were highly susceptible to colitis, indicating a critical role for AGR2 in protection from disease. We conclude that AGR2 is a unique member of the PDI family, with a specialized and nonredundant role in intestinal mucus production.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- van Anken E, Braakman I. Versatility of the endoplasmic reticulum protein folding factory. Crit Rev Biochem Mol Biol. 2005;40:191–228. - PubMed
-
- Persson S, et al. Diversity of the protein disulfide isomerase family: Identification of breast tumor induced Hag2 and Hag3 as novel members of the protein family. Mol Phylogenet Evol. 2005;36:734–740. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

