Transcription factor E2-2 is an essential and specific regulator of plasmacytoid dendritic cell development
- PMID: 18854153
- PMCID: PMC2631034
- DOI: 10.1016/j.cell.2008.09.016
Transcription factor E2-2 is an essential and specific regulator of plasmacytoid dendritic cell development
Abstract
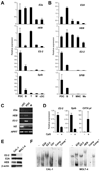
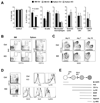
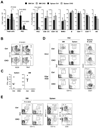
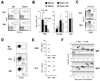
Plasmacytoid dendritic cells (PDCs) represent a unique immune cell type specialized in type I interferon (IFN) secretion in response to viral nucleic acids. The molecular control of PDC lineage specification has been poorly understood. We report that basic helix-loop-helix transcription factor (E protein) E2-2/Tcf4 is preferentially expressed in murine and human PDCs. Constitutive or inducible deletion of murine E2-2 blocked the development of PDCs but not of other lineages and abolished IFN response to unmethylated DNA. Moreover, E2-2 haploinsufficiency in mice and in human Pitt-Hopkins syndrome patients was associated with aberrant expression profile and impaired IFN response of the PDC. E2-2 directly activated multiple PDC-enriched genes, including transcription factors involved in PDC development (SpiB, Irf8) and function (Irf7). These results identify E2-2 as a specific transcriptional regulator of the PDC lineage in mice and humans and reveal a key function of E proteins in the innate immune system.
Figures


References
-
- Amiel J, Rio M, de Pontual L, Redon R, Malan V, Boddaert N, Plouin P, Carter NP, Lyonnet S, Munnich A, et al. Mutations in TCF4, encoding a class I basic helix-loop-helix transcription factor, are responsible for Pitt-Hopkins syndrome, a severe epileptic encephalopathy associated with autonomic dysfunction. Am J Hum Genet. 2007;80:988–993. - PMC - PubMed
-
- Banchereau J, Pascual V. Type I interferon in systemic lupus erythematosus and other autoimmune diseases. Immunity. 2006;25:383–392. - PubMed
-
- Barchet W, Cella M, Colonna M. Plasmacytoid dendritic cells--virus experts of innate immunity. Semin Immunol. 2005;17:253–261. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
- P01 AI078869/AI/NIAID NIH HHS/United States
- T32 GM007367/GM/NIGMS NIH HHS/United States
- T32 HD055165/HD/NICHD NIH HHS/United States
- HHMI/Howard Hughes Medical Institute/United States
- AI007525/AI/NIAID NIH HHS/United States
- R01 AI072571/AI/NIAID NIH HHS/United States
- R37 AI026918/AI/NIAID NIH HHS/United States
- R21 AI085439/AI/NIAID NIH HHS/United States
- GM007367/GM/NIGMS NIH HHS/United States
- F31 AI080184/AI/NIAID NIH HHS/United States
- AI072571/AI/NIAID NIH HHS/United States
- HD055165/HD/NICHD NIH HHS/United States
- AI078869/AI/NIAID NIH HHS/United States
- AI080184/AI/NIAID NIH HHS/United States
- F31 AI066459/AI/NIAID NIH HHS/United States
- T32 AI007525/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

