A transcriptome study of the QseEF two-component system and the QseG membrane protein in enterohaemorrhagic Escherichia coli O157 : H7
- PMID: 20056703
- PMCID: PMC2889445
- DOI: 10.1099/mic.0.033027-0
A transcriptome study of the QseEF two-component system and the QseG membrane protein in enterohaemorrhagic Escherichia coli O157 : H7
Abstract
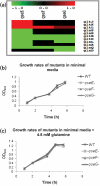
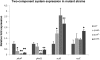
QseE is a sensor kinase that responds to epinephrine, sulfate and phosphate. QseE constitutes a two-component signalling system together with the QseF sigma(54)-dependent response regulator. Encoded within the same operon as qseEF is the qseG gene, which encodes a membrane protein involved in the translocation of a type III secretion effector protein of enterohaemorrhagic Escherichia coli (EHEC) into epithelial cells. The qseEGF genes also form an operon with the glnB gene, which encodes the E. coli nitrogen sensor PII protein. Here we report a transcriptome analysis comparing qseE, qseF andqseG single mutants with the wild-type strain. This study revealed that the proteins encoded by these genes play a modest but significant role in iron uptake. Although QseEFG regulate genes involved in nitrogen utilization, these proteins do not play a notable role in nitrogen metabolism. In addition, QseEFG regulate transcription of the rcsBC and phoPQ two-component systems, linking several signal transduction pathways. The similarity of the microarray profiles of these mutants also indicates that these proteins work together. These data indicate that QseEFG are involved in the regulation of virulence and metabolism in EHEC.
Figures

References
-
- Anonymous (1997). Applied Biosystems Prism 7700 Sequence Detection System: User Bulletin #2. Norwalk, CT: Perkin-Elmer Corp.
-
- Bader, M. W., Sanowar, S., Daley, M. E., Schneider, A. R., Cho, U., Xu, W., Klevit, R. E., Le Moual, H. & Miller, S. I. (2005). Recognition of antimicrobial peptides by a bacterial sensor kinase. Cell 122, 461–472. - PubMed
-
- Bolstad, B. M., Irizarry, R. A., Astrand, M. & Speed, T. P. (2003). A comparison of normalization methods for high density oligonucleotide array data based on variance and bias. Bioinformatics 19, 185–193. - PubMed
-
- Campellone, K. G., Robbins, D. & Leong, J. M. (2004). EspFU is a translocated EHEC effector that interacts with Tir and N-WASP and promotes Nck-independent actin assembly. Dev Cell 7, 217–228. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

