Endometrial senescence is mediated by interleukin 17 receptor B signaling
- PMID: 39010112
- PMCID: PMC11247761
- DOI: 10.1186/s12964-024-01740-5
Endometrial senescence is mediated by interleukin 17 receptor B signaling
Erratum in
-
Correction: Endometrial senescence is mediated by interleukin 17 receptor B signaling.Cell Commun Signal. 2024 Jul 22;22(1):370. doi: 10.1186/s12964-024-01754-z. Cell Commun Signal. 2024. PMID: 39039545 Free PMC article. No abstract available.
Abstract
Background: We previously identified Il17RB, a member of the IL17 superfamily, as a candidate marker gene for endometrial aging. While IL17RB has been linked to inflammation and malignancies in several organ systems, its function in the endometrium has not been investigated and is thus poorly understood. In the present study, we performed a functional analysis of this receptor with the aim of determining the effects of its age-associated overexpression on the uterine environment.
Methods: We analyzed IL17RB-related signaling pathways and downstream gene expression in an immortalized human endometrial glandular epithelial cell line ("hEM") forced to express the receptor via lentiviral transduction ("IL17RB-hEM"). We also prepared endometrial organoids from human endometrial tissue sourced from hysterectomy patients ("patient-derived EOs") and exposed them to cytokines that are upregulated by IL17RB expression to investigate changes in organoid-forming capacity and senescence markers. We analyzed RNA-seq data (GEO accession number GSE132886) from our previous study to identify the signaling pathways associated with altered IL17RB expression. We also analyzed the effects of the JNK pathway on organoid-forming capacity.
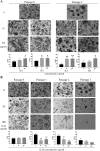
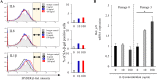
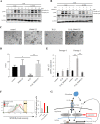
Results: Stimulation with interleukin 17B enhanced the NF-κB pathway in IL17RB-hEM, resulting in significantly elevated expression of the genes encoding the senescence associated secretory phenotype (SASP) factors IL6, IL8, and IL1β. Of these cytokines, IL1β inhibited endometrial organoid growth. Bioinformatics analysis showed that the JNK signaling pathway was associated with age-related variation in IL17RB expression. When IL17RB-positive cells were cultured in the presence of IL17B, their organoid-forming capacity was slightly but non-significantly lower than in unexposed IL17RB-positive cells, but when IL17B was paired with a JNK inhibitor (SP600125), it was restored to control levels. Further, IL1β exposure significantly reduced organoid-forming capacity and increased p21 expression in endometrial organoids relative to non-exposure (control), but when IL1β was paired with SP600125, both indicators were restored to levels comparable to the control condition.
Conclusions: We have revealed an association between IL17RB, whose expression increases in the endometrial glandular epithelium with advancing age, and cellular senescence. Using human endometrial organoids as in vitro model, we found that IL1β inhibits cell proliferation and leads to endometrial senescence via the JNK pathway.
Keywords: Aging; Endometrial senescence; IL17B; IL17RB; IL1β; JNK; Macrophage; NF-κB; Organoid; SASP.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Katagiri Y, Jwa SC, Kuwahara A, Iwasa T, Ono M, Kato K, Kishi H, Kuwabara Y, Harada M, Hamatani T, Osuga Y. Assisted reproductive technology in Japan: a summary report for 2019 by the ethics committee of the Japan society of obstetrics and gynecology. Reprod Med Biol. 2022;21:e12434. 10.1002/rmb2.12434 - DOI - PMC - PubMed
-
- Franasiak JM, Forman EJ, Hong KH, Werner MD, Upham KM, Treff NR, Scott RT Jr. The nature of aneuploidy with increasing age of the female partner: a review of 15,169 consecutive trophectoderm biopsies evaluated with comprehensive chromosomal screening. Fertil Steril. 2014;101:656–e663651. 10.1016/j.fertnstert.2013.11.004 - DOI - PubMed
-
- Rubio C, Bellver J, Rodrigo L, Castillón G, Guillén A, Vidal C, Giles J, Ferrando M, Cabanillas S, Remohí J, et al. In vitro fertilization with preimplantation genetic diagnosis for aneuploidies in advanced maternal age: a randomized, controlled study. Fertil Steril. 2017;107:1122–9. 10.1016/j.fertnstert.2017.03.011 - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

