Combined deletion of Glut1 and Glut3 impairs lung adenocarcinoma growth
- PMID: 32571479
- PMCID: PMC7311173
- DOI: 10.7554/eLife.53618
Combined deletion of Glut1 and Glut3 impairs lung adenocarcinoma growth
Abstract
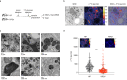
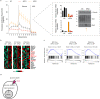
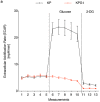
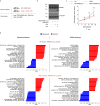
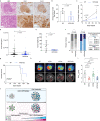
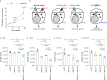
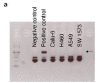
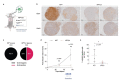
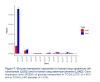
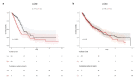
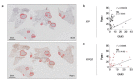
Glucose utilization increases in tumors, a metabolic process that is observed clinically by 18F-fluorodeoxyglucose positron emission tomography (18F-FDG-PET). However, is increased glucose uptake important for tumor cells, and which transporters are implicated in vivo? In a genetically-engineered mouse model of lung adenocarcinoma, we show that the deletion of only one highly expressed glucose transporter, Glut1 or Glut3, in cancer cells does not impair tumor growth, whereas their combined loss diminishes tumor development. 18F-FDG-PET analyses of tumors demonstrate that Glut1 and Glut3 loss decreases glucose uptake, which is mainly dependent on Glut1. Using 13C-glucose tracing with correlated nanoscale secondary ion mass spectrometry (NanoSIMS) and electron microscopy, we also report the presence of lamellar body-like organelles in tumor cells accumulating glucose-derived biomass, depending partially on Glut1. Our results demonstrate the requirement for two glucose transporters in lung adenocarcinoma, the dual blockade of which could reach therapeutic responses not achieved by individual targeting.
Keywords: NanoSIMS; cancer biology; genetically engineered mouse model of cancer; glucose transporters; human; lamellar bodies; lung adenocarcinoma; mouse.
© 2020, Contat et al.
Conflict of interest statement
CC, PA, NZ, SS, JP, SE, LJ, CG, BL, ML, RG, AR, SB, CN, IZ, SC, GK, JR, EA, AM, EM No competing interests declared
Figures







References
-
- Bankhead P, Loughrey MB, Fernández JA, Dombrowski Y, McArt DG, Dunne PD, McQuaid S, Gray RT, Murray LJ, Coleman HG, James JA, Salto-Tellez M, Hamilton PW. QuPath: open source software for digital pathology image analysis. Scientific Reports. 2017;7:16878. doi: 10.1038/s41598-017-17204-5. - DOI - PMC - PubMed
-
- Commisso C, Davidson SM, Soydaner-Azeloglu RG, Parker SJ, Kamphorst JJ, Hackett S, Grabocka E, Nofal M, Drebin JA, Thompson CB, Rabinowitz JD, Metallo CM, Vander Heiden MG, Bar-Sagi D. Macropinocytosis of protein is an amino acid supply route in Ras-transformed cells. Nature. 2013;497:633–637. doi: 10.1038/nature12138. - DOI - PMC - PubMed
-
- Faget J, Groeneveld S, Boivin G, Sankar M, Zangger N, Garcia M, Guex N, Zlobec I, Steiner L, Piersigilli A, Xenarios I, Meylan E. Neutrophils and snail orchestrate the establishment of a Pro-tumor microenvironment in lung Cancer. Cell Reports. 2017;21:3190–3204. doi: 10.1016/j.celrep.2017.11.052. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

