T cell self-reactivity during thymic development dictates the timing of positive selection
- PMID: 33884954
- PMCID: PMC8116051
- DOI: 10.7554/eLife.65435
T cell self-reactivity during thymic development dictates the timing of positive selection
Abstract
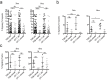
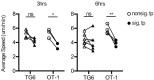
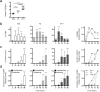
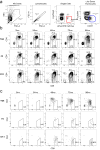
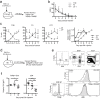
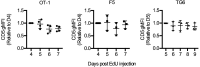
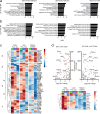
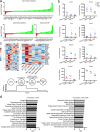
Functional tuning of T cells based on their degree of self-reactivity is established during positive selection in the thymus, although how positive selection differs for thymocytes with relatively low versus high self-reactivity is unclear. In addition, preselection thymocytes are highly sensitive to low-affinity ligands, but the mechanism underlying their enhanced T cell receptor (TCR) sensitivity is not fully understood. Here we show that murine thymocytes with low self-reactivity experience briefer TCR signals and complete positive selection more slowly than those with high self-reactivity. Additionally, we provide evidence that cells with low self-reactivity retain a preselection gene expression signature as they mature, including genes previously implicated in modulating TCR sensitivity and a novel group of ion channel genes. Our results imply that thymocytes with low self-reactivity downregulate TCR sensitivity more slowly during positive selection, and associate membrane ion channel expression with thymocyte self-reactivity and progress through positive selection.
Keywords: TCR; developmental biology; immunology; inflammation; ion channels; mouse; positive selection; self-reactivity; thymocyte.
© 2021, Lutes et al.
Conflict of interest statement
LL, ZS, LM, SP, JK, AH, SA, AS, NY, ER No competing interests declared
Figures








References
-
- Barrett T, Wilhite SE, Ledoux P, Evangelista C, Kim IF, Tomashevsky M, Marshall KA, Phillippy KH, Sherman PM, Holko M, Yefanov A, Lee H, Zhang N, Robertson CL, Serova N, Davis S, Soboleva A. “NCBI GEO: Archive for Functional Genomics Data Sets - Update.”. Nucleic Acids Research. 2013;41:gks1193. doi: 10.1093/nar/gks1193. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

