Vasculogenic skin reprogramming requires TET-mediated gene demethylation in fibroblasts for rescuing impaired perfusion in diabetes
- PMID: 39604331
- PMCID: PMC11603198
- DOI: 10.1038/s41467-024-54385-w
Vasculogenic skin reprogramming requires TET-mediated gene demethylation in fibroblasts for rescuing impaired perfusion in diabetes
Abstract
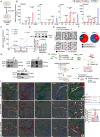
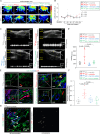
Tissue nanotransfection (TNT) topically delivers Etv2, Foxc2, and Fli1 (EFF) plasmids increasing vasculogenic fibroblasts (VF) and promoting vascularization in ischemic murine skin. Human dermal fibroblasts respond to EFF nanoelectroporation with elevated expression of endothelial genes in vitro, which is linked to increased ten-eleven translocase 1/2/3 (TET) expression. Single cell RNA sequencing dependent validation of VF induction reveals a TET-dependent transcript signature. TNTEFF also induces TET expression in vivo, and fibroblast-specific EFF overexpression leads to VF-transition, with TET-activation correlating with higher 5-hydroxymethylcytosine (5-hmC) levels in VF. VF emergence requires TET-dependent demethylation of endothelial genes in vivo, enhancing VF abundance and restoring perfusion in diabetic ischemic limbs. TNTEFF improves perfusion and wound closure in diabetic mice, while increasing VF in cultured human skin explants. Suppressed in diabetes, TET1/2/3 play a critical role in TNT-mediated VF formation which supports de novo blood vessel development to rescue diabetic ischemic tissue.
© 2024. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures



References
-
- Larouche, J. & Aguilar, C. A. New technologies to enhance in vivo reprogramming for regenerative medicine. Trends Biotechnol.37, 604–617 (2019). - PubMed
-
- Graf, T. & Enver, T. Forcing cells to change lineages. Nature462, 587–594 (2009). - PubMed
-
- Wingo, M. & Rafii, S. Endothelial reprogramming for vascular regeneration: past milestones and future directions. Semin. Cell Dev. Biol.122, 50–55 (2022). - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- BioProject/PRJNA1175408
Grants and funding
- W81XWH-21-1-0033, W81XWH-22-1-0146, HT94252410131/United States Department of Defense | United States Army | Army Medical Command | Congressionally Directed Medical Research Programs (CDMRP)
- R01 DK136814/DK/NIDDK NIH HHS/United States
- R01 DK135447/DK/NIDDK NIH HHS/United States
- R01 DK141513/DK/NIDDK NIH HHS/United States
- U01 DK119099/DK/NIDDK NIH HHS/United States
- R01 DK125835/DK/NIDDK NIH HHS/United States
- R01 DK128845/DK/NIDDK NIH HHS/United States
- DK141513, DK128845, DK135447, DK125835, DK119099, DK136814/U.S. Department of Health & Human Services | NIH | National Institute of Diabetes and Digestive and Kidney Diseases (National Institute of Diabetes & Digestive & Kidney Diseases)
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

